U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-06-133 Date: March 2007 |
A number of laboratory studies (Stark et al., 1993; Stokes et al., 2000) have demonstrated that treating ASR-affected concrete with lithium compounds can reduce or eliminate future expansion due to ASR (e.g., figure 19). Typically, such studies have used laboratory-sized specimens with relatively small cross-sections and it has not yet been demonstrated that lithium treatment is effective with larger specimens that are more representative of elements of concrete structures.
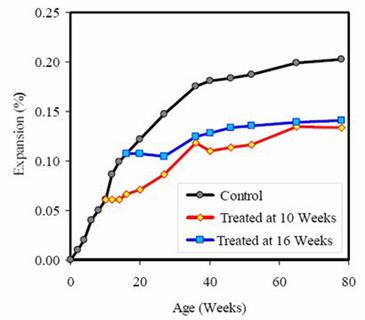
Figure 19. Expansion of concrete prisms after treatment with
lithium at 10 weeks (expansion = 0.061 percent) and 16 weeks
(expansion = 0.107 percent) (Thomas and Stokes, 2004).
Numerous structures have been treated by spraying the surface of the structure with a solution of lithium (both LiNO3 and LiOH have been used). These structures have included pavements, bridge decks and other bridge components, and median barriers. The solution has been applied by either truck-mounted spraying systems (figure 20) or hand-held pressurized spray bottles (figure 21).

Figure 20. Spraying 30 percent LiNO3 solution with a tanker
truck on a concrete pavement near Mountain Home, ID.

Figure 21. Spraying 30-percent LiNO3 solution with handheld
spray applicator on barrier wall near Leominster, MA.
Typical application rates have been in the range of 0.12 to 0.24 liters per square meter (L/m2) (3 to 6 gallons per square feet (gal /1000 ft2)). The most commonly used lithium compound for this purpose is a 30 percent LiNO3 solution. Commercially available solutions contain a proprietary surfactant to enhance penetration.
There are few data available regarding the depth of lithium penetration following lithium treatment. Figure 22 shows lithium concentration profiles in concrete cores cut from a pavement after six treatments (one treatment in each of spring and fall for 3 consecutive years) of 0.24 L/m2 (6 gal/1000 ft2). The depth of lithium penetration is clearly dependent on the extent of cracking.
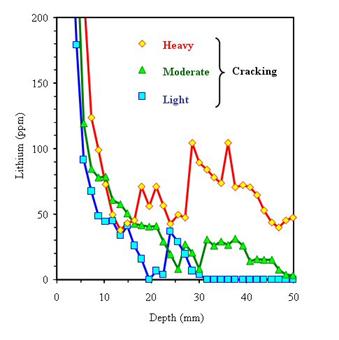
1 mm = 0.039 inch
Figure 22. Lithium concentration profiles for concrete pavement after six treatments
(at approximately 6-month intervals) of 0.24 L/m2 (6 gal/1000 ft2) (Stokes et al., 2002).
Figure 22 indicates that very little lithium penetrates below 25 to 50 mm (1 to 2 inches) unless the concrete is heavily cracked. Even in heavily cracked concrete, the lithium concentration at this depth is low, and its ability to suppress ASR is questionable[3].
Most of the structures that have been treated topically with lithium have not been monitored properly (i.e., other than by simple visual inspection) to confirm whether the treatment has been effective in terms of suppressing ASR expansion.
Electrochemical impregnation techniques have been used to increase lithium penetration on a number of structures (Whitmore and Abbot, 2000). A typical setup (i.e., for a bridge deck) is shown in figure 24 and includes the following parameters:
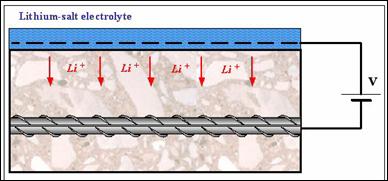
Figure 23. Electrochemical lithium impregnation.
Two such cases of using this electrochemical technology have been documented in the literature; these are two bridge decks, one in Arlington, VA, the other in Seaford, DE. In both cases, lithium borate was used as the electrolyte. Cores were taken from the deck in Virginia after 8 weeks of electrochemical treatment. Slices taken from the cores and subjected to chemical analysis revealed the data shown in
table 11.
Table 11. Penetration of lithium after electrochemical
treatment of bridge deck.
| Depth of slice (mm) | Lithium (ppm) |
|---|---|
6-19 |
315-343 |
19-32 |
203-265 |
1 mm = 0.039 inch
The data indicate that significant lithium penetrates to a depth of at least 19 to 32 mm (0.75 to 1.25 inches), and these dosages are theoretically high enough to have a beneficial effect on reducing ASR-induced expansion (see footnote 2 in chapter 3).
In March 2006, two columns in Houston, TX, were selected for electrochemical treatment as part of the Federal Highway Association Lithium Implementation Technology Program. Figure 24 shows the process of the treatment for one of the columns. The entire treatment was completed mid-May of 2006.
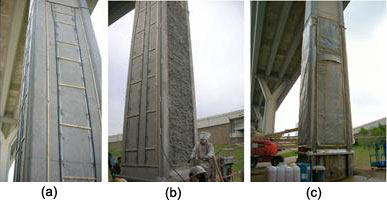
Figure 24. Electrochemical lithium treatment process. (a) irrigation tubes, wood splices, and metal strips are placed on the column. The metal strips are attached to titanium mesh that runs inside holes drilled into the sides of the column. (b) A cellulose layer is applied to the side of the column, and (c) plastic sheeting is placed on all sides of the column. The gutters under the sheeting collect excess lithium for reuse.
Vacuum impregnation is an alternative to pressure injection and has been used to increase grout penetration into cracked concrete. A number of structures have been treated with lithium using this technique; these include several substructure elements (beams and columns) of the New Jersey Turnpike, a number of elements on the Prospect Avenue Viaduct in Johnstown, PA, a trapezoidal prestressed bridge girder (treated by vacuum impregnation as part of a study of ASR-mitigation methods on 5 girders in Corpus Christi, TX), and sections of a barrier wall on Highway 2 near Leominster, MA (figure 26).
At the time of writing, no data were available concerning the depth of lithium penetration as a result of vacuum impregnation.
|
 |
Figure 25. Typical vacuum impregnation setup.
Before treating a structure with lithium-based compounds, an investigation should be conducted to ensure that the structure meets the following criteria:
Lithium treatment will not "repair" any damage that has already occurred. A protocol for selecting structures that may be suitable for lithium-treatment is available from FHWA (FHWA-RD-04-113).
Treating structures with lithium is a technology that is still under development and, at this time, recommended protocols for selecting the type of treatment (e.g., topical, electrochemical, or vacuum impregnation) or methodologies for performing the treatment do not exist. Electrochemical and vacuum impregnation require specialized knowledge and equipment, and should be conducted only by an experienced contractor. Topical applications are relatively simple to perform, and a few general guidelines are provided in table 12. Figure 26 shows examples of LiNO3 precipitation after application.
Table 12. General guidelines for topical lithium treatment.
| Treatment Procedure |
|---|
|
The number of individual treatments that can be applied to a structure will be governed by economics and other aspects of the repair strategy. For example, if the structure is being treated prior to the application of a concrete or asphalt overlay, there may only be time for a single treatment. For pavements or bridge decks that remain exposed after treatment, additional treatments may be considered at appropriate intervals. For example, the treatment of State Route 1 in Delaware involved a total of 6 individual treatments over a 3-year period. |
As the efficacy of lithium treatment has yet to be established, it is recommended that treated structures are tested and monitored properly. Some suggestions for monitoring are provided in table 13. Figure 27 shows crack mapping and length change monitoring.
The authors are not aware of any studies aimed at evaluating the effect of lithium nitrate on the environment.
Figure 26. Precipitation of LiNO3 from solution (a) on barrier wall and (b) on pavement.
Table 13. Suggestions for monitoring lithium-treated structures.
| Monitoring Guidelines |
|---|
|
Figure 27. Monitoring techniques-(a) crack mapping of a
barrier wall and (b) measuring length changes on
concrete pavement with a DEMEC gauge.