U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Federal Highway Administration > Publications > Research > Structures > Critical Literature Review of High-Performance Corrosion Reinforcements in Concrete Bridge Applications |
Publication Number: FHWA-HRT-4-093 |
Previous | Table of Contents | Next
Within the present context, CRR is defined as reinforcing steel that exhibits improved corrosion behavior in chloride contaminated concrete compared to conventional bare, black steel.[31] Accordingly, primary emphasis is placed on stainless steels and modified stainless steels, including propriety alloys and stainless steel clad bars that are presently available.
General
The term stainless steel generically refers to iron base alloys with a minimum of 12 weight percent (w/o) Cr. At this Cr concentration, a passive film self-forms on air and water exposure. In addition, common alloying elements include Ni, Mo, N, Ti, and perhaps others. Except for martensitic stainless steels, carbon is an impurity. Stainless steels that are being considered for reinforcing concrete are of either a ferritic, austenitic, or duplex (ferrite plus austenite) microstructure according to the phase(s) present, which, in turn, is determined by composition. As such, Cr is a ferrite (bcc) stabilizer, whereas Ni stabilizes austenite (fcc). Other classes of stainless steel are martensitic (noted above), super-ferritic, super-austenitic, and precipitation-hardenable. For various reasons, including excessive strength (martensitic and precipitation-hardenable), poor corrosion resistance (martensitic), and higher than necessary alloying element concentrations and, hence, cost (super-ferritic and super-austenitic), the super-ferritic, super-austenitic, and precipitation-hardenable classes are not considered viable reinforcement candidates.
Ferritic stainless steels typically have less than 17 w/o Cr and little or no Ni. For austenitics, Cr is 18-20 w/o, and Ni is 8-10 w/o. Other alloying elements also may be present (for example, 2 and 3 w/o Mo for American Iron and Steel Institute (AISI) Types 316 and 317, respectively). Ferritic-austenitic stainless steels, termed duplexes, contain 22-28 w/o Cr and 4-8 w/o Ni. This yields a microstructure comprised of both ferrite and austenite, typically at a 50-50 ratio. The super-ferritics typically contain ~25-30 w/o Cr and <4 w/o Ni and the super-austenitics ~20 w/o Cr, and 18-25 w/o Ni.
Stainless steel has been employed as concrete reinforcement in Canada, Denmark, Germany, Italy, Japan, Mexico, South Africa, the United States, and the United Kingdom. While this reinforcement has been used throughout some structures, stainless steel has more generally been limited to construction joints or critical gaps between columns and decks.
Relevant Material Properties
The relevant material properties for reinforcing corrosion-resistant or conventional steel are mechanical, corrosion, weldability, thermal, magnetic, and economic. Mechanical properties are addressed by applicable standards and design codes, whereas the other properties are more subjective and addressed by the designer in conjunction with materials selection considerations.
Corrosion Behavior of Stainless Steels
Although the literature addressing corrosion of stainless steels is voluminous, it pertains largely to acid and seawater rather than highly alkaline applications. Much emphasis has been placed on identifying the alloy (of which there are hundreds) that provides adequate corrosion resistance for the application in question (in the present context, reinforced concrete bridges) at minimal cost.
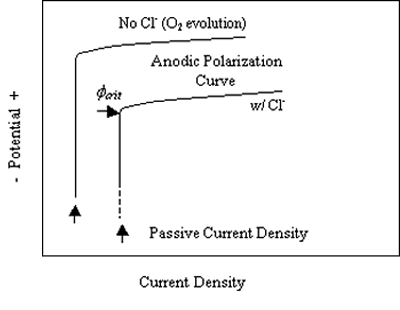
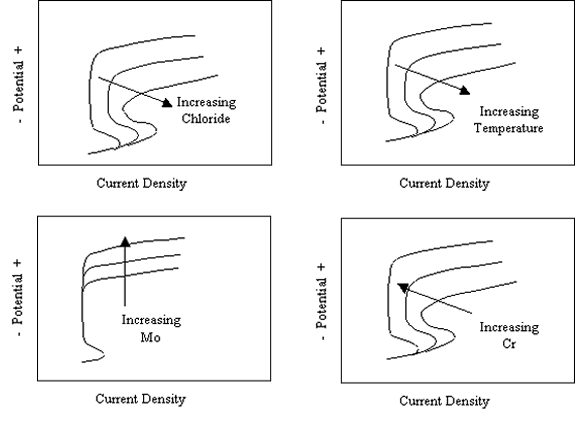
The most common forms of corrosion for stainless steels are localized (pitting, crevice, and intergranular) and environmental cracking. Of these, pitting is the most relevant form for reinforcing steel inconcrete structures. Resistance to pitting attack historically has been characterized by any one of several parameters, including (1) critical pitting potential, Øcrit, (2) critical pitting temperature (CPT) and (3) the pitting resistance equivalent (PRE or, alternatively, pitting resistance equivalent number (PREN)). The first of these, Øcrit, is defined as the least positive potential for which pitting occurs. This is illustrated schematically in figure 3 for the case of an electrode such as stainless steel exposed to aqueous solutions with and without Cl-. Here, the two anodic polarization curves are characterized by passivity and a relatively low, potential independent current density regime at negative potentials that transitions at more positive potentials to high current densities. The passive current density is lower, and the transition to higher current density occurs at a more positive potential in the absence of Cl-. This current density increase is a consequence of O2 evolution, not corrosion. With Cl-, however, the current density transition results from the onset of pitting. Figure 4 shows schematically how the anodic polarization curve and Øcrit are affected by temperature, Cl- concentration, and alloy composition. Thus, the passive domain is compromised by increasing temperature and Cl- concentration, and expanded by increased Mo concentration.
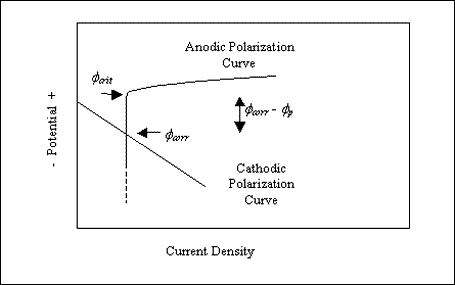
A critical aspect of pitting resistance is the magnitude of the difference between the critical pitting potential (Øcrit) and the corrosion potential (Øcorr), as illustrated schematically by figure 5. Thus, pitting should not occur in situations where Øcorr remains negative to Øcrit and vice versa. Table 1 illustrates this by comparing these two parameters based on 4.25 years' exposure of various alloys in seawater, and figure 6 plots Øcrit - Øcorr versus weight loss, where the latter parameter indicates the extent of pitting corrosion.[32]
The finding that resistance to pitting decreases with increasing temperature (figure 4) is the basis for defining this resistance in terms of a critical temperature; that is, a temperature below which the passive film does not break down locally, and above which it does. This temperature is determined experimentally by polarizing the metal or alloy in question at a constant potential more positive than Øcorr and progressively increasing temperature. The onset of a current density increase then defines the CPT.
|
Alloy Designation |
Øcrit, VSCE |
Range for Øcorr, VSCE |
|---|---|---|
|
Type 430 SS |
-0.130 |
-0.310 to 0.230 |
|
Type 304 SS |
-0.020 |
-0.140 to 0.280 |
|
Type 316 SS |
0.100 |
0.090 to 0.385 |
|
Carpenter 20 CbTM |
0.05 |
0.120 to 0.520 |
|
Incoloy 825TM |
0.525 |
0.180 to 0.530 |
|
Hastelloy CTM |
>0.900 |
0.530 |
|
SS: Stainless steel. |
||
Although the difference between Øcrit and Øcorr and the CPT indicate resistance to pitting, as explained above, the PRE/PREN more often is employed for materials selection purposes for austenitic and duplex stainless steels. This parameter is calculated from the expression
| (11) |
where A typically ranges from 6 to 30, with a value of 16 being commonly employed for duplex stainless steels and 30 for austenitics. As such, the PRE/PREN is based solely on composition of three alloying elements. Also, note the relatively strong influence of N, followed by Mo. PRE values in excess of 40 generally are considered necessary to avoid pitting and crevice corrosion in ambient seawater. A lesser value should suffice for stainless steels in concrete because of the relatively high pore water pH and the corrosion inhibiting role of OH-, as discussed above.
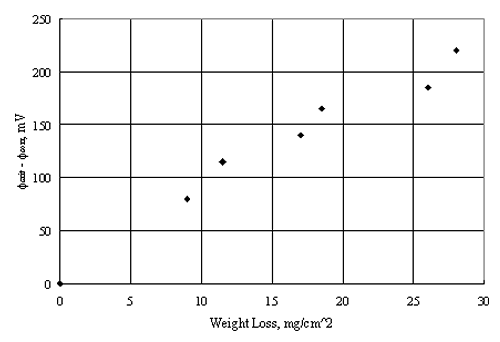
Table 2 lists PRE/PREN values for several common stainless steels. Although the PRE/PREN provides a quantitative measure of performance, it does not consider the effect of other alloying elements, impurities, and microstructure, all of which can be important. In addition, equation 11 has evolved from a wide base of experience in acid processing fluid exposures and not for steel in concrete. While the general qualitative nature of the expression is expected to apply in concrete as well, the coefficients may be different.
|
Stainless Alloy |
PRE/PREN (A = 16) |
|---|---|
|
Type 430 |
17 |
|
Type 304 |
18 |
|
Type 316 |
24 |
|
Type 316LN |
26 |
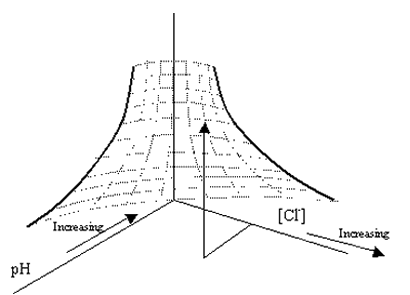
As noted above, the critical pitting potential increases with increasing pH because OH- serves as a passivator. Figure 7 provides a schematic illustration of the combined pH and Cl- effect, such that a surface is generated corresponding to Øcrit. With increasing PRE/PREN, this surface of Øcrit values is displaced toward more positive potentials.
Pitting Mechanism
Theories of passivity fall into two general categories, one based on adsorption and the other on presence of a thin oxide film. Pitting in the former case arises as detrimental or activator species, such as Cl-, compete with O2 or OH- at specific surface sites. By the oxide film theory, detrimental species become incorporated into the passive film, leading to its local dissolution or to development of conductive paths. Once initiated, pits propagate auto-catalytically according to the generalized reaction,
| (12) |
resulting in acidification of the active region and corrosion at an accelerated rate (M+n and M are the ionic and metallic forms of the corroding metal). As evidence of this process, acidic pH values are routinely measured at actively corroding sites on reinforcing steel upon breaking open Cl- contaminated concrete irrespective of the alkaline pore water.[1]
Particularly noteworthy is the pitting mechanism proposed by Galvele[33,34] and Muller and Gavele.[35] This is predicated on the anodic process (equation 2) occurring at the base of a cylindrical pit or similar geometrical feature and supported by an Na salt containing electrolyte followed by hydrolysis (equation 12). Pit stability requires maintaining a critical [H+], designated pHcrit. This was assumed as the value at which the oxide (passive) film is in equilibrium with dilute metal ions in solution based on the reaction
| (13) |
where
| (14) |
with Ks being the solubility product. The model demonstrated that a stable pHcrit resulted when the product of pit depth and current density achieved a critical value. Thus, both generating Me+n via the anodic reaction and confining the H+ hydrolysis product (as occurs with increasing pit depth or surface irregularities and an associated restriction on outward diffusion and electromigration of cations), facilitate pitting. s
Galvele reasoned further that Øcrit is the most negative potential at which pHcrit can be maintained at the metal-solution interface. This was expressed analytically as
| (15) |
where h if magnitude of the positive (anodic) polarization from Øcorr, Øinh is the potential shift associated with presence of any inhibitor, and Ø is the potential gradient in the pit.
The concept that the criterion for pit initiation is that the product of current density (ic) and surface feature depth (x) achieve a critical value has important implications for stainless steels in corrosive service, including those of reinforcing steel in concrete. This arises because surface treatments such as thermal-mechanical processing, welding, and pickling all affect surface roughness. Accordingly, pitting susceptibility, as reflected by Øcrit or by CPT (although not by PRE/PREN) should vary in proportion to surface roughness that arises from a particular processing or treatment.