U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| TECHBRIEF |
| This techbrief is an archived publication and may contain dated technical, contact, and link information |
| Publication Number: FHWA-HRT-14-040 Date: March 2014 |
Publication Number: FHWA-HRT-14-040 Date: March 2014 |
PDF files can be viewed with the Acrobat® Reader®
FHWA Publication No.: FHWA-HRT-14-040 FHWA Contacts: Paul Virmani, HRDI-60, (202) 493-3052, paul.virmani@dot.gov and Hamid Ghasemi, HRDI-60, (202) 493-3042, hamid.ghasemi@dot.gov. |
This document is a technical summary of the Federal Highway Administration report, An FHWA Special Study:
Post-Tensioning Tendon Grout Chloride Thresholds (FHWA-HRT-14-039).
INTRODUCTION
Since the 1970s, the number of pre-tensioned and post-tensioned (PT) concrete bridge structures utilizing high-strength seven-wire strands has increased steadily. For bonded PT tendons, the grout functions as the last layer of corrosion protection for the highly stressed seven-wire strands by providing a high pH environment to form a protective oxide film on the strand surface and also acting as a physical barrier to water, oxygen, and chloride ions. Corrosion of strands initiates when the protective oxide film is compromised due to chloride attack or carbonation of the surrounding grout upon exposure to water and air. Once corrosion starts, it propagates at a certain rate controlled by many factors such as oxygen availability, moisture content, electrical resistance, grout pH, and chloride concentration. In this TechBrief, a prestressed seven-wire strand will be simply referred to as a “PT strand.”
Corrosion problems have been observed in some PT bridges in Florida, Virginia, and Minnesota mainly due to grout voids, poor quality grout, water infiltration, and duct holes/cracking. These deficiencies can lead to severe corrosion or failure of the PT tendons. A recent discovery of a grout product with elevated levels of chloride used in a PT concrete straddle cap located in Corpus Christi, TX, resulted in a full investigation of a potential corrosion problem. Chloride concentrations were reported to be as high as 5.27 percent by weight of cement.(1, 2) These numbers exceed the current limits set by all domestic and international regulatory committees. For example, the chloride limits for prestressed concrete imposed by American Concrete Institute committees are either 0.06 percent water-soluble chloride by weight of cement or 0.08 percent acid-soluble chloride by weight of cement. These chloride limits are arbitrary concentrations determined by the code authorities and specifications after taking into account a factor of safety. Conversely, chloride threshold is the actual amount of chloride needed to initiate corrosion. There is limited information reported in the literature pertaining to the chloride threshold of the PT tendons. As chloride information is needed to assess corrosion risk of the PT bridges containing the chloride contaminated grout, the Federal Highway Administration sponsored a 6-month accelerated corrosion testing program to determine chloride threshold(s) of PT strands exposed to chloride-contaminated grout. This TechBrief explains how two chloride threshold values of 0.4 and 0.8 percent by weight of cement were determined for corrosion initiation and corrosion propagation, respectively, using supporting test results.
Test Specimens
A 6-month accelerated corrosion testing program employed three types of laboratory specimens as shown in figure 1 through figure 3. The first type was single king (i.e., center) wire specimens that were extracted from the regular PT strands. More than 50 specimens were tested in aqueous solutions having a low pH of 9 to simulate carbonated grout and a high pH of 13.6 to simulate highly alkaline normal grout, each with 10 chloride concentrations. Some of them were stressed at 60 percent guaranteed ultimate tensile strength (GUTS), and the others were placed in a no stress condition. The second type was 12-inch-long single-strand specimens embedded in grout. A total of 40 specimens were fabricated, and some were stressed at 60 percent GUTS and/or contained an artificial gout void. The effects of stress, grout void, and chloride concentration were to be evaluated in an ideal condition.
The third type was 10-ft-long multi-strand specimens encased in steel loading frames. A total of eight specimens were prepared to evaluate the effects of stress, void, and chloride concentration in the macro-cell corrosion condition, which has been responsible for the observed severe corrosion problems in the field. To create simulated macro-cell corrosion, each specimen contained four strands stressed at 60 percent GUTS and was passed through an artificial void near the top anchor plate. This served as the macro-anode. Additionally, a bundle of five 7-ft-long unstressed strands were completely buried in grout, which served as the macro-cathode. The grout mixes were admixed with eight acid-soluble chloride concentrations ranging from 0 to 2.0 percent by weight of cement.

Figure 1. Photo. Single-wire specimen.

Figure 2. Photo. Single-strand specimens.

Figure 3. Photo. Multi-strand specimens.
Test Conditions
The single-wire specimens were tested in an ambient laboratory condition. The single-strand and multi-strand specimens were placed in two environmental chambers and went through a full exposure cycle of 104 °F and 90 percent relative humidity (RH) (hot and humid (H & H)) to 77 °F and 60 percent RH (ambient) to 14 °F and 40 percent RH (freezing and dry (F & D)) to 77 °F and 60 percent RH (ambient). Each sub-cycle lasted 2 weeks, and three full cycles were repeated during the 6-month testing period. In the middle of the exposure testing, a water recharging event was simulated by adding 0.17 fl oz of distilled water to some of the voided single-strand specimens and 5.1 fl oz to all of the multi-strand specimens.
Performance Evaluation
Corrosion performance data were collected weekly to monitor progressive changes in the specimens. Corrosion potential data indicated thermodynamic state of corrosion to indicate whether corrosion initiation was possible. Corrosion kinetics indicating how fast corrosion progressed was determined by corrosion rate data (single-wire and single-strand specimens) or macro-cell corrosion current density data (multi-strand specimens). In addition, apparent grout resistivity (single-strand specimens) and apparent grout resistance (multi-strand specimens) were measured to determine conductivity of the grout which can be related to corrosiveness of the environment. Upon completion of the 6-month accelerated corrosion testing, an autopsy of the specimens embedded in the grout was carried out followed by a thorough corrosion damage assessment of the extracted strands. Actual damage conditions observed on individual specimens exposed to eight chloride concentrations were compared to the corresponding non-destructively collected data of the specimens. From this, chloride threshold values were determined.
MAJOR FINDINGS
The corrosion rate of the single wires tested in pH 13.6 solutions was less than 0.05 mil/year at chloride concentrations up to 0.6 percent and increased at higher chloride concentrations. For the specimens tested in pH 9.0 solutions containing 0.04 percent and higher chloride concentrations, typical active corrosion behavior was observed—corrosion potentials became more negative, and corrosion rates increased. These test results suggest that PT strands can tolerate chloride contamination without significant corrosion up to 0.6 percent by weight of cement in carbonation-free (high pH) grout, whereas as low as 0.04 percent chloride by weight of cement can initiate active corrosion in the carbonated (low pH) grout. This is below the American Association of State Highway and Transportation Officials specified chloride limit of 0.08 percent by weight of cement.(3) Once corrosion starts in the low pH environment, the level of corrosion damage depends on chloride concentration.
For single-strand specimens, chloride concentration of 0.4 percent by weight of cement was the lowest concentration to make the strands more prone to corrosion in most conditions. Mean corrosion potential gradually became negative starting from 0.4 percent chloride. Mean corrosion rate also became progressively higher starting from 0.4 percent chloride. Dependence of corrosion rate on temperature was observed (i.e., higher corrosion rate in H & H cycles and negligible corrosion rate in F & D cycles regardless of chloride concentration). The limited data suggest that the stressed strands exposed to water in the grout void may be more susceptible to corrosion than the unstressed ones if chloride concentration is higher than 0.08 percent and the risk of corrosion is elevated with increased chloride concentration. All control specimens experienced steadily decreasing corrosion rates as their corrosion potential data also indicated the passive behavior.
The first rust spot (considered a sign of corrosion initiation) observed on the single-strand specimens began to appear when chloride concentration was 0.4 percent. Measurable pits deeper than 2 mil (considered a sign of corrosion propagation and also the lowest measurement limit of a digital pit gauge) were observed when chloride concentration was 0.8 percent. Figure 4 through figure 7 show physical conditions of the corroding wires removed from 0.4 and 0.8 percent chloride concentration specimens, respectively. The red arrow in figure 4 indicates a rust stain matching with the corrosion spot marked in the red circle. As chloride concentration increased, severity of the corrosion worsened, particularly for the stressed specimens with void. Mean pit depths ranged between 3.6 and 6.2 mil. A combination of a void, recharged water, and elevated chloride concentration can be detrimental, evidenced by the markedly higher number of pits found on the stressed 2.0 percent chloride specimen with void.
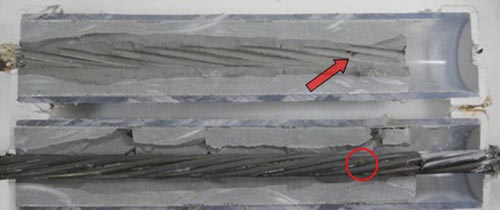
Figure 4. Photo. Pitting corrosion on a 0.4 percent chloride concentration single-strand specimen.

Figure 5. Photo. Close-up view of cleaned rust spot on a 0.4 percent chloride concentration single-strand specimen.

Figure 6. Photo. Pitting corrosion on a 0.8 percent chloride concentration single-strand specimen.

Figure 7. Photo. Second view of pitting corrosion on a 0.8 percent chloride concentration single-strand specimen.
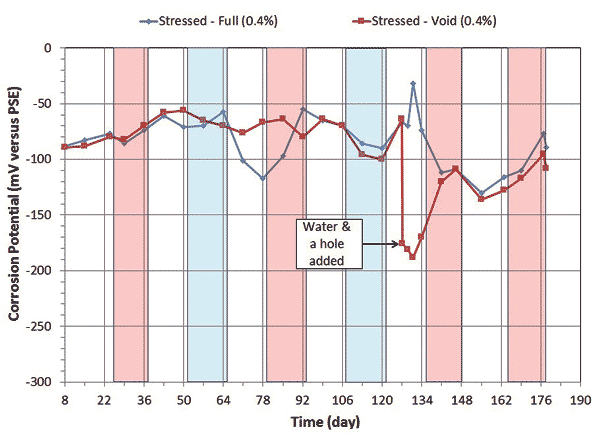
Corrosion potential and corrosion rate data of single-strand specimens containing 0.4 and 0.8 percent chloride concentrations are shown in figure 8 through figure 11.
PSE = Pseudo-reference electrode.
Figure 8. Graph. Electrochemical data of 0.4 percent chloride concentration single-strand specimens: corrosion potential versus time.
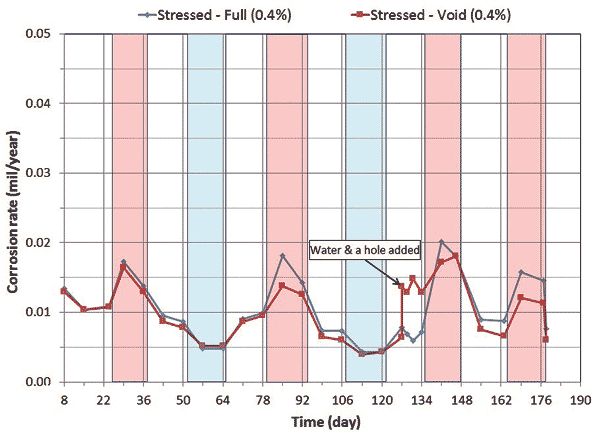
Figure 9. Graph. Electrochemical data of 0.4 percent chloride concentration single-strand specimens: corrosion rate versus time.
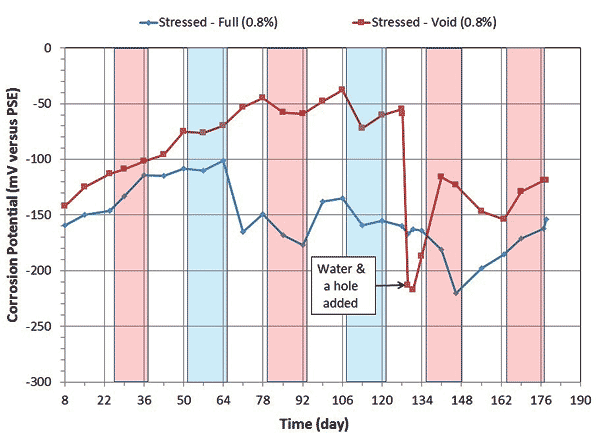
Figure 10. Graph. Electrochemical data of 0.8 percent chloride concentration single-strand specimens: corrosion potential versus time.
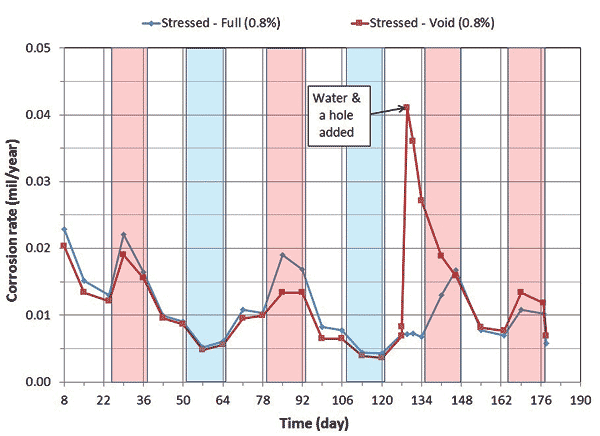
Figure 11. Graph. Electrochemical data of 0.8 percent chloride concentration single-strand specimens: corrosion rate versus time.
For multi-strand specimens, chloride concentration was a more influential parameter for corrosion potential than exposure condition. As the macro-cell corrosion setup intended, stressed strands exhibited more negative corrosion potentials than unstressed ones, making the stressed strands anodic to the unstressed ones at any chloride concentration. It is unclear if presence of stress or localized corrosion at the void/grout interface or both made the entire stressed strands active. When chloride concentration increased to 0.4 percent, mean corrosion potentials of the stressed strands decreased to the 90th percentile of corrosion probability. Mean macro-cell corrosion current density also began to increase at 0.4 percent chloride. Figure 12 and figure 13 show the mean corrosion potential data of the stressed strands and mean macro-cell corrosion current density data, respectively.
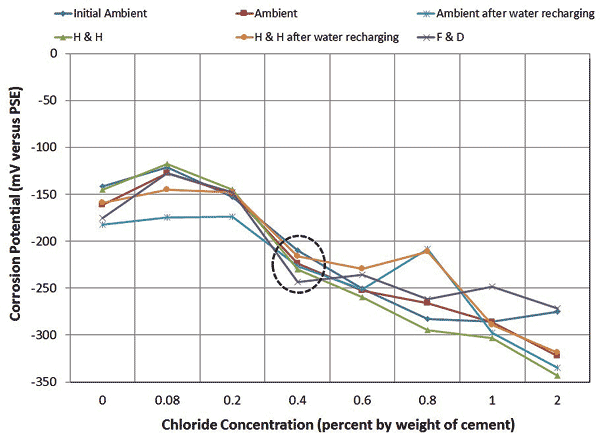
Figure 12. Graph. Electrochemical data of multi-strand specimens: mean corrosion potentials.
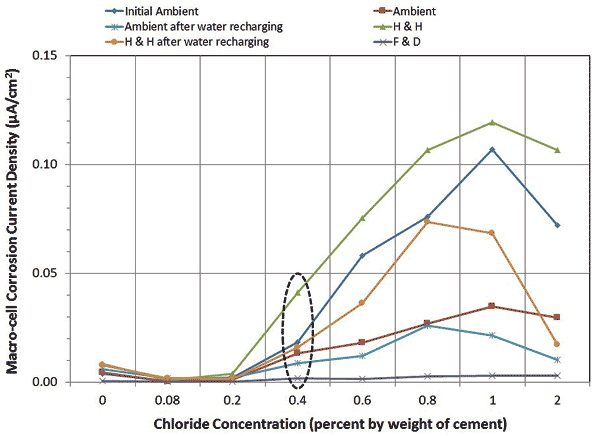
Figure 13. Graph. Electrochemical data of multi-strand specimens: mean macro-cell corrosion current densities.
Even though it was not included, the unstressed strands exhibited active corrosion potential at 2.0 percent chloride concentration. The H & H and F & D cycles produced the highest and the lowest mean macro-cell corrosion current densities, respectively, independent of chloride concentration. Water recharging did not influence the mean corrosion potentials and mean macro-cell current densities significantly.
An autopsy of the multi-strand specimens revealed that, in general, stressed segments started to show superficial rust at 0.4 percent chloride concentration and heavy rust requiring acid cleaning at 0.8 percent chloride concentration. This observation could be made with both of the void/grout interface segments and in-grout segments. Mean pit depths measured on the stressed in-grout strands increased from 4.3 to 9.4 mil when chloride concentration increased from 0.4 to 2.0 percent. For unstressed strands, superficial rust began to show up at 0.6 percent chloride concentration, and none of the pits exceeded 2 mil regardless of chloride concentration. Figure 14 and figure 15 show the numbers of pits counted in the in-grout segment of stressed and unstressed strands and mean pit depths of the stressed strands as a function of chloride concentration, respectively.
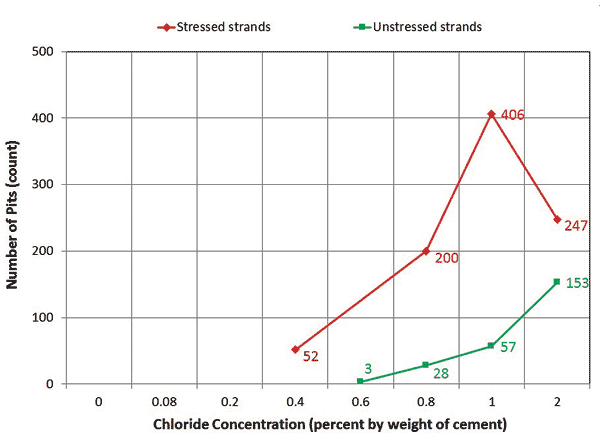
Figure 14. Graph. Number of pits measured on multi-strand specimens.
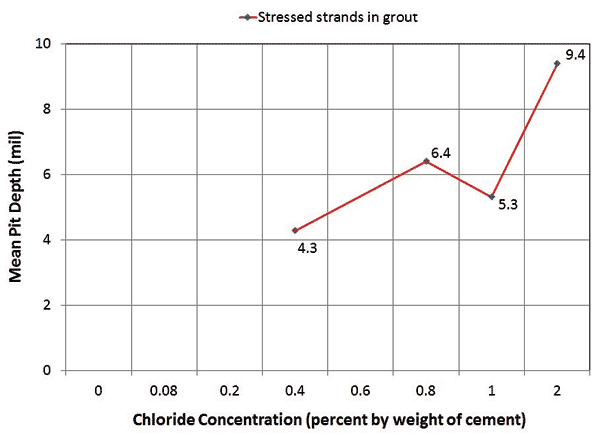
Figure 15. Graph. Mean pit depths measured on multi-strand specimens.
For the interface segments, mean pit depth was approximately 6 mil when chloride concentration was less than 1.0 percent. At 2.0 percent chloride concentration, mean pit depth increased to 11 mil. The largest measured pit depths were 60 and 36 mil for the interface segments and in-grout segments, respectively. Figure 16 and figure 17 show pitting corrosion damage found on 0.4 and 0.8 percent chloride concentration multi-strand specimens, respectively. At 0.4 percent chloride concentration, the mean pit depth was 4.1 mil with a maximum pit depth of 9.0 mil. At 0.8 percent chloride concentration, mean pit depth was 5.9 mil with a maximum pit depth of 11 mil.

Figure 16. Photo. Pitting corrosion observed on a stressed wire of 0.4 percent chloride concentration multi-strand specimen.

Figure 17. Photo. Pitting corrosion observed on a stressed wire of 0.8 percent chloride concentration multi-strand specimen.
Crevice corrosion, another form of localized corrosion, was also observed on many of the multi-strand specimens containing 0.4 percent and higher chloride concentrations. Similar to the observed pitting corrosion, crevice-induced corrosion got worse as chloride concentration increased. Figure 18 and figure 19 show typical crevice corrosion damage found on 0.4 and 0.8 percent chloride specimens, respectively. At 0.4 percent chloride concentration, mean pit depth was 4.5 mil with a maximum pit depth of 8 mil. At 0.8 percent chloride concentration, mean pit depth was 4.5 mil with a maximum pit depth of 6 mil.

Figure 18. Photo. Crevice corrosion between two stressed wires on 0.4 percent chloride concentration multi-strand specimen.

Figure 19. Photo. Crevice corrosion between two stressed wires on 0.8 percent chloride concentration multi-strand specimen.
A general trend observed from the multi-strand specimens with normal grout at the interface is that more pits were found on the segments closer to the interface when chloride concentration was 0.8 and 1.0 percent. When chloride concentration was between 0.08 and 0.6 percent, no measurable pits were found at all. This finding suggests that stressed strands near the interface were subjected to the most intensive macro-cell corrosion if chloride concentration was 0.8 percent chloride. At this level of chloride, a small surface area of the interface became the most anodic in the presence of abundant dissolved oxygen and moisture in the void. The number of pits tended to decrease as a segment was further away from the interface. It is thought that this phenomenon was related to weakened macro-cell corrosion current in the deeper grout. Mean pit depth data did not follow the same trend in that it was fairly uniform around 6 mil along the most part of the stressed strand length including the interface section.
CONCLUSIONS
The corrosion process of PT strands is divided into two stages: corrosion initiation and corrosion propagation. Based on the test results obtained through a 6-month accelerated corrosion testing program and a subsequent autopsy of the specimens, two chloride threshold values, one for each stage, were determined for the PT strands fully encased in normal grout.
The first threshold is 0.4 percent chloride concentration by weight of cement, which is the lowest amount to initiate corrosion of strands. At this threshold, rust spots formed, and small number of pits started to form beneath some of the rust spots. It is a conservative number, and there is still sufficient time to mitigate future corrosion problems if necessary. Therefore, it can serve as the lower limit in assessing longer-term corrosion risk of PT bridges.
The second critical chloride threshold is found in association with corrosion propagation. At this threshold, corrosion started to intensify in term of number of pits and pit depth. Test data and autopsy results obtained in this study indicated that 0.8 percent chloride by weight of cement is the critical threshold. This threshold should be considered as the upper limit in corrosion risk assessment. Once chloride concentration exceeds the critical threshold, significant corrosion damage can occur rapidly, and structural integrity of the PT bridges may be compromised in the near future. The actual deterioration rate can be influenced by many factors such as moisture content, temperature, oxygen availability, and grout resistivity.
It should be emphasized that the threshold values are applicable to normal grout condition only. They may not be adequate in other conditions such as carbonated grout, segregated grout, duct cracks, grout voids filled with water with or without chloride ions, and free sulfate ions in contact with the strands. In these circumstances, corrosion should start below the proposed threshold values.
REFERENCES
Researchers—This study was performed by the Center for Advanced Infrastructure and Transportation of Rutgers University, 100 Brett Road, Piscataway, NJ 08854. Distribution—The TechBrief is being distributed according to a standard distribution. Direct distribution is being made to the Divisions and Resource Center. Availability—This TechBrief may be obtained from the FHWA Product Distribution Center by e-mail to report.center@dot.gov, fax to (814) 239-2156, phone to (814) 239-1160, or online at https://www.fhwa.dot.gov/research. Key Words— Chloride threshold, Chloride, Corrosion initiation, Corrosion propagation, Seven-wire strand, Post-tensioning, Grout, Autopsy, Accelerated corrosion testing, Sulfate ions, Void. Notice—This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The U.S. Government assumes no liability for the use of the information contained in this document. The U.S. Government does not endorse products or manufacturers. Trademarks or manufacturers’ names appear in this report only because they are considered essential to the objectives of the document. Quality Assurance Statement—The Federal Highway Administration (FHWA) provides high-quality information to serve the Government, industry, and public in a manner that promotes public understanding. Standards and policies are used to ensure and maximize the quality, objectivity, utility, and integrity of its information. FHWA periodically reviews quality issues and adjusts its programs and processes to ensure continuous quality improvement. |