U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-07-039
Date: July 2007 |
||||||
Chapter 1 - Corrosion Resistant Alloys for Reinforced Concrete1. INTRODUCTIONThe United States has a major investment in its highway system, the operational performance of which, in conjunction with that of other transportation modes, is critical to the Nation’s economic health and societal functionality. While deterioration of structures with time is a normal and expected occurrence, the rate at which this has occurred for highway bridges since advent in the 1960s of a clear roads policy, as affected by winter application of deicing salts in northern locations, has been abnormally advanced and has posed significant challenges, both economically and technically. Also important is similar advanced deterioration of reinforced concrete bridges in coastal locations, both northern and southern, as a consequence of sea water or spray exposure. In both cases (deicing salts and marine exposure), the deterioration is a consequence of the aggressive nature of the chloride ion in combination with moisture and oxygen.1 Over half of the total bridge inventory in the United States is of the reinforced concrete type, and these structures have proved to be particularly susceptible. A recent study2 has indicated that the annual direct cost of corrosion to bridges is $5.9 to $9.7 billion. If indirect factors are included also, this cost can be as much as 10 times higher.3 As this problem has manifested itself during the past 40 or so years, technical efforts have been directed towards, first, understanding the deterioration mechanism and, second, developing prevention and intervention strategies. With regard to the former, steel and concrete are in most aspects mutually compatible, as exemplified by the fact that, in the absence of chlorides, the relatively high pH of concrete pore solution (pH ≈ 13.0-13.8) promotes formation of a protective oxide (passive) film such that corrosion rate is negligible and decades of relatively low maintenance result. In the presence of chlorides, even at concentrations at the steel depth as low as 0.6 kilograms per cubic meter (kg/m3) (1.0 pound per cubic yard (lb/yd3)) (concrete weight basis),4 the passive film may become locally disrupted and active corrosion commence. Once this occurs, solid corrosion products form progressively near the steel-concrete interface and lead ultimately to concrete cracking and spalling. Figure 1.1 shows a photograph illustrating such damage for the case of a coastal bridge piling. Because corrosion induced deterioration is progressive, inspections for damage assessment must be performed routinely; and present Federal guidelines require a visual inspection every 2 years.5 If indicators of deterioration are not addressed, then public safety is at risk. As an example, corrosion induced concrete spalls occur as potholes in a bridge deck and contribute to unsafe driving conditions. In the extreme, structural failure and collapse result. Figure 1.1. Photo. Cracked and spalled marine bridge piling.  Corrosion induced deterioration of reinforced concrete can be modeled in terms of three component steps: (1) time for corrosion initiation, Ti, (2) time, subsequent to corrosion initiation, for appearance of cracking on the external concrete surface (crack propagation), Tp, and (3) time for surface cracks to progress into further damage and develop into spalls, Td, to the point where, if repairs and rehabilitations are not performed, the functional service life, Tf, is reached.6 Figure 1.2 illustrates these parameters schematically as a plot of cumulative damage versus time. Of the three component terms, Ti normally occupies the longest period and, as such, is the predominant factor in determining useful service life. Based upon the corrosion deterioration model represented by figure 1.2, methods of life-cycle cost analysis (LCCA) are now commonly employed to evaluate and compare different materials selection and design alternatives. This approach considers both initial cost and the projected life history of maintenance, repair, and rehabilitation that are required until the design life is reached. These are evaluated in terms of the time value of money, from which present worth is determined; and comparisons between different options can then be made on a cost normalized basis. In the early 1970s, research studies were performed that qualified epoxy-coated reinforcing steel (ECR) as an alternative to black bar;7,8 and for the past 30 years ECR has been specified by most State Departments of Transportation (DOTs) for bridge decks and substructures exposed to chlorides. At the same time, ECR was augmented by use of low water-to-cement ratio (w/c) concrete, possibly with pozzolans or corrosion inhibitors (or both), and concrete covers of 65 millimeters (mm) or more.9 However, premature corrosion induced cracking of marine bridge substructures in Florida10,11,12,13 indicated that ECR is of little benefit for this type of exposure; and while performance of ECR in northern bridge decks has been generally good to-date (30- plus years), still the degree of corrosion resistance afforded in the long term for major structures with design lives of 75 to 100 years is uncertain. In response to this, interest has focused during the past decade upon more corrosion resistant alternatives to ECR, stainless steels in particular. Such corrosion resistant steels become particularly competitive on a life-cycle cost basis, since the higher initial expense of the steel per se may be recovered over the life of the structure via reduced repairs and rehabilitations. Figure 1.2. Schematic illustration. Various steps in deterioration of reinforced concrete due to chloride induced corrosion. 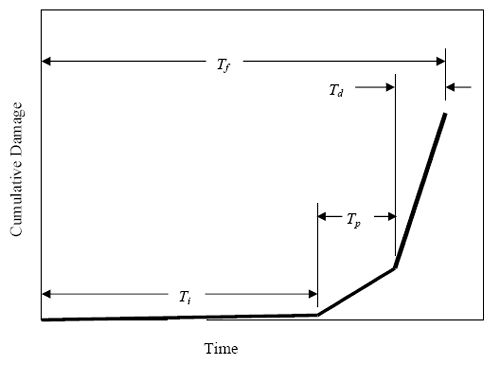 The present research study is being performed jointly by Florida Atlantic University and the Florida Department of Transportation as a 6-year effort to evaluate the suitability of various corrosion resistant reinforcements for bridges exposed to chlorides. An initial phase of the study provided a critical literature review of corrosion resistant reinforcements.14 The present report details research findings during the initial 3 years of the project.
|