U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-04-150 Date: July 2006 |
Previous | Table of Contents | Next
Various methods of specimen preparation are used, depending on the nature of the specimen and the information to be obtained. Smoothly lapped specimens are required for quantitative examination with the stereomicroscope. Small loose particles and many fracture surfaces do not require extensive preparation. Examination with the transmitted-light microscopes (i.e., the petrographic and P/EF microscopes) requires very thin specimens mounted on glass or very small grains of the specimen mounted in an index of refraction oil or epoxy. The fluorescence features of the P/EF microscope necessitate that the specimen not only be very thin, but also be impregnated with an epoxy containing a fluorescent dye. The features of large specimens of aggregate rock can be displayed best through sawing, lapping, and etching. It is often necessary to crush such material for use in specific tests. Bulk specimens of sand, gravel, and crushed aggregate usually require a reduction in overall volume and separation into size-graded fractions.
There are three types of slices prepared for stereomicroscopic examination: (1) basic lapped slices, (2) vertical sections of horizontal slices (or vice versa), and (3) acid-etched slices.
The determination of the components, ingredient boundaries, reaction products, and parameters of the air-void system requires the use of a stereomicroscope (see figure 19). The types of items perceivable with the stereomicroscope are described in chapter 8. Because it is impossible to focus clearly on a rough surface at the magnifications required for the petrographic examination of HCC with a stereomicroscope, it is necessary that smooth (finely lapped) surfaces be prepared on sawed slices of the specimens. The smoother the sawcut, the easier the lapping routine will be.
Unless specific precautions are taken to avoid it, undercutting is common during the production of finely lapped surfaces. It is the process in which softer constituents are removed more rapidly than harder constituents so that the harder material (usually the aggregate) stands up higher than the softer components (see figure 41). During the lapping process, the slice rides on the high, hard portions and grinding compound collects under the soft portions. Hard aggregate standing above an undercut paste has a rounded edge, and the area of visible aggregate is increased. There is no sharp line or change in surface texture at the point where the theoretical flat-lapped surface changes to a slope down into the paste. No exercise of logic or mental reconstruction of what the surface should be will allow an accurate determination of the paste-aggregate boundary or the paste-aggregate ratio in such a slice.
Figure 41. Undercutting.
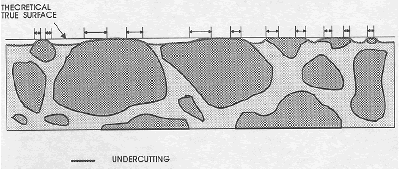
Where the surface falls below the theoretical true surface, the paste-aggregate boundary is rounded over and cannot be defined by a view from above, as through a microscope. Arrows indicate the length of each questionable area.
Undercutting is very common on surfaces that are finished on soft polishing laps. It can also occur on hard cast-iron laps, but may be greatly minimized with the use of sufficient weight on the back of the specimen to hold the specimen closer to the lap and to create additional wear on the high areas. If the specimen is riding on pieces of high, hard aggregate, the buildup of grinding compound in the low, soft portions of the specimen will erode this softer material. This can cause a general rounding of the surface contours with ensuing enlargement of voids, as well as the loss of sharpness and clarity on the edges of cracks and other features. It can also reduce the distinction at the boundaries of differing components and can lead to a loss of secondary reaction products.
Producing a lapped slice is a five-step process, as listed in table 13. Additional details on the preparation of surfaces for microscopical analysis may be found in many references (e.g., ASTM C 856 (petrographic examination) and ASTM C 457 (determination of air-void systems)).
|
Cut a slice of the HCC from the specimen according to the plan for petrographic analysis as devised in chapter 3. The oil-cooled saw produces smoother surfaces that require less lap time than do surfaces produced with a water-cooled saw. As mentioned in chapter 3, label all fragments of the original specimen to identify their origin and, whenever possible, their orientation. A slice thickness of 15 mm is recommended; however, inspect the material after the first cut to make sure that a thicker slice is not required because of the presence of a previously unseen crack or other weakness. If the specimen crumbles or cracks into several fragments after the first sawcut, you may have to cement the specimen back together with a strong epoxy before you can shape a slice.
The slice must usually be trimmed to fit the slice holder of the lapping device. Most holders are circular to allow free rotation. Be careful not to trim away any portion of the area of greatest interest to the client. Often the portion of greatest interest will be the top, or wearing, surface. In such cases, any trimming will have to be done on the bottom. If there are no obvious differences to be seen between the top and the bottom of the core and the section does not exceed the diameter of the specimen-holding ring of the lap (150 mm for VTRC equipment) in any dimension, a lapped section can be produced that will be representative of the full depth of the specimen. Very often the lowest 20 to 25 mm or so does not require examination unless it is very different from the upper portions.
Very fragile specimens may require impregnation. A number of materials and techniques for impregnation are available. Carnauba wax is recommended in ASTM C 457; however, it must be used with safety precautions to prevent explosion during heating toremove excess wax. Roberts and Scali (1984) recommended the use of a 1:5 solution of colorless nylon fingernail hardener in acetone. Methanol may be used as an alternate solvent with the nail hardener. They prefer the commercial nail hardener to a solution of pure nylon because some of the softening ingredients in the commercial preparation are valuable in preventing chipping. The nylon solution may be applied to the HCC at any stage of the lapping as seems necessary. The hardener is brushed on the surface until it fills the cracks and thoroughly coats the interior of the voids. The hardener is allowed to dry before lapping is continued. Fragile HCC may require the use of the hardener during all stages of the lapping; other HCCs will require the hardener only during the last portion of the lapping procedures. Before examination and determination of the air–void parameters, the hardener should be removed by soaking in the appropriate solvent until the plastic shine is dissolved. The choice of an impregnating agent should include consideration of the desirability and difficulty of removing the impregnating agent from the surface to be examined.
If epoxy or another permanent substance is used for impregnation, it will become a lasting part of the specimen, make detection of subtle textural differences in the HCC more difficult in reflected light, and obscure the viewing of reaction products (in voids) and internal surfaces. This may or may not be important, depending on the purposes of the investigation for which the specimen is being prepared. Usually in cases where impregnation is necessary because of extreme friability of the HCC, the examination of the paste microstructure by transmitted light or an SEM will be called for and the impregnation will be beneficial for these examinations. Various techniques may be used to impregnate HCC slices with numerous types of substances. LR White, a one-part, extremely low–viscosity embedding material, has been found to work very well. To provide contrast in optical microscopy, it can be dyed with a solution of methylene blue in ethanol.
Once you are satisfied that the surface has been made as flat as possible with the coarsest abrasive and all saw marks are gone, continue to work on the two mechanical laps with the automatic abrasive feed (figure 8). Place the specimen slice in the slotted specimen holder on the lap. Adjust the yokes so that the grinding slurry does not drop inside the slotted specimen holders and the specimens rotate freely as the lap moves beneath them. Place the cover plate on the back of the specimen and place any weights on top of the cover plate. Load the abrasive cup with about 20 ml of the appropriate abrasive and fill it about three-quarters full of lapping oil. Set the clock for 20 minutes (min) of lapping. Inspect the specimens and make a judgment concerning the next abrasive. Each abrasive should be finer than the last one used and should be used repeatedly until the marks from the coarser abrasive are gone. A good system is to use one lap for all but the finest abrasive and change to the other lap for the finest one. Lap the surface with successively finer abrasives (see list in chapter 2) until it is suitable for microscopical observation.
| CAUTION: If the cleaning of lapped slices is not done promptly, the volatile oils will evaporate and the grinding compound deposits will cake and become hard to remove. Therefore, do not allow a specimen that requires cleaning to lose volatile oils or solvents by evaporation. |
| CAUTION: Use of ultrasonic cleaning equipment may be harmful to the surface of concrete specimens; therefore, such treatment should not be used without care and experimentation (see ASTM C 457). |
| WARNING: Do not immerse any portion of your hand in an ultrasonic bath while the ultrasound is on. The cavitation caused by the ultrasound can erode fingernails and damage skin. |
If cleaning of lapped slices must be delayed, submerge the slice in a solvent such as isopropyl alcohol (99 percent). Caked grinding compound can make viewing of the interior surfaces and the crystal forms of the paste and reaction products impossible and can contribute to undercutting and destruction of the specimen. A few minutes of ultrasonic cleaning in solvent may be used for cleaning lapped specimens.
At VTRC, we have not experienced any loss of reaction products or loosening of the aggregates or other surface degradation because of brief ultrasonic cleaning. As an alternative to ultrasonic cleaning, the specimen can be carefully cleaned of grinding compound and loose particles with a soft brush (soft as a baby’s hairbrush) while it is immersed in a safety-approved container of isopropyl alcohol. It has been reported that a fine, high–velocity spray of solvent (used in an efficient hood) will clean lapped slices efficiently. This procedure has not been tried at VTRC, and we have no evidence concerning the proper pressure and velocity of the spray that would be required to clean without causing damage. Whatever cleaning method is used, as much grinding compound and debris as possible should be removed without damaging the surface or any reaction products in the voids.
| WARNING: Persons unfamiliar with the hazards of these cleaning compounds are referred to the Chemical Safety Data Sheets published by the Manufacturing Chemists Association, Inc., 1825 Connecticut Avenue, NW, Washington, DC 20009, or the Materials Safety Data Sheet that may be obtained from the supplier of the compound. |
If the day is reasonably bright, scan the light from a window as it is reflected at a very low angle from a finely lapped slice. It should be possible to discern objects such as buildings and trees (see figure 44).
Alternatively, hold the slice so that it reflects light from an overhead light fixture. It should be possible to see the small details of the diffusion cover of the light or even discern the presence of writing on the light bulb. The edges of the sections of the air voids will be sharp and not eroded or crumbled, air–void sections as small as 10 µ in diameter will be clearly distinguishable at magnifications of less than 150X, and all cracks will be easily visible and free of debris.
The lapped surface needs to show all reaction products, the inner surface of the voids, the hydration, etc., in an as-received condition. At VTRC, space and technician time arepremium commodities, and the slice used for the air–void determination must be the same surface used for all microscopic evaluations of the concrete sampled and is usually the only slice kept in the archives.
If difficulty is encountered in preparing a lapped surface of the proper quality, a common cause is a weak cement paste matrix. The problem is manifested by the plucking of sand grains from the surface during lapping, with consequent scratching of the surface (as the loose aggregate moves under the surface being prepared) and undercutting of the pastearound the harder aggregate particles. A second cause of difficulty may be friable particles of aggregate. For any quantitative analyses, areas that are scratched or imperfect indicate the need for additional preparation of the specimen. Often, additional cleaning and more time on the free-abrasive laps are all that is required.
Figure 42. Well-prepared surface viewed in ordinary illumination.

1 inch = 25.4 mm
Exhibits the quality of the surfaces required for microscopical examination and analyses.
Figure 43. Well-prepared surface viewed at a very low angle toward a source of illumination (an illuminated screen).

The quality of the reflection of a distant object (here, the quality of the reflection of the reversed word SURFACE on the screen) indicates the flatness of the lapped surface.
Figure 44. Properly finished slice (same as that shown in figures 42 and 43).

Reflection of outdoor objects as seen on a bright day. The camera was focused on the reflection; thus, to the camera, the slice is out of focus. The human eye sees both in focus.
If a horizontal slice was taken from a core to study changes in the air-void system (or other feature) with depth, it may later be desirable to lap finely and examine a vertical slice of one or more of the lapped slices already available (or vice versa). In such a case, the slice in question must be secured in an upright position in a mounting medium so that a slice that is at right angles to the direction of the first slice can be cut and lapped (see figures 45 and 46).
Etching is performed to enhance the differences in acid resistance or solubility that might exist between the phases of a rock or HCC. Some of the phase differences that the petrographer might wish to enhance are rhombic dolomite crystals in micritic–carbonate rock, fly–ash particles andcarbonated areas in HCC, and aggregate particles in HCC paste. Lapped slices of concrete andlapped surfaces on carbonate rock specimens are often etched to make visible and emphasize various phase differences and phase boundaries in the specimen. These differences may be the contrasts between aggregate and paste, various mineralogical differences, and even the differences caused by the depositional and metamorphic history of the rock.
Figure 45. Slice cut at right angles to original slice: Edges of thin top surfaces of a concrete core embedded in epoxy and repotted in mortar in a mold 152 mm in diameter and then resliced and finely lapped to allow examination of the vertical surface.
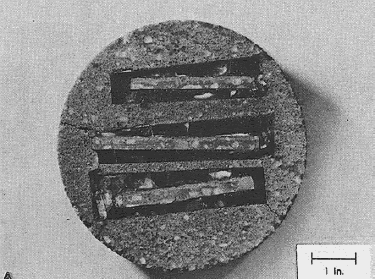
1 inch = 25.4 mm
In this case, the concrete was slightly etched with weak HCl to delineate the depth of carbonation.
Figure 46. Slice cut at right angles to original slice: Left portion of middle slice shown in figure 45 is enlarged.

1 inch = 25.4 mm
The carbonated area is the area above the lower edge of the whiter area near the wearing surface.
The etching of finely lapped specimens of HCC must be delayed until after the specimen has been thoroughly examined and any analysis of the percentage and shape of any constituents that might be changed by the etching is completed (e.g., air). If the etching is being performed to aid in the determination of the paste content for use in the formulae for air–void parameters, the etching and paste determination must be performed on the same surface as was the air-content determination.
Procedures for acid–etching of slices are outlined in table 14. Before proceeding with etching,ensure that the slice is free of oil and, if heated, allow it to cool to room temperature. The oil used as a lubricant for lapping must have been removed from the slice by long exposure or oven treatment. Steam must not form in the HCC; therefore, the temperature in the oven must not exceed 90° C.
Etching portion of slice:
|
Etching entire slice:
|
Thin sections must be thin enough for sufficient light to pass through them to enable the microscopist to observe the specimen using the particular features of the microscopes (see chapters 12 and 13). Thus, the sections should be as thin as is practical.
Instructions for fabricating standard thin sections from rock for examination with the petrographic microscope are available in many good books on optical mineralogy or petrology and are usually included with any equipment purchased for this purpose. Special instructions for preparing thin sections of HCC, thin sections for study with fluorescent light, and thin sections of particular aggregates are included here. Additional information, particularly covering the preparation of large-area thin sections, can be found in Concrete Petrography: A Handbook of Investigative Techniques(St. John, et al., 1998).
The thickness of the thin sections fabricated for the usual geologic/petrologic/mineralogic examination of rocks and minerals is 25 to 30 µm. If rock or rocklike specimens are sent to a company that fabricates thin sections without further instructions, the thin sections produced will be 25 to 30 µm. For the determination of the component minerals in coarse–grained igneous and metamorphic rocks, this thickness generally works fairly well. Some rock–oriented petrographers depend on thin sections being a standard thickness so that they can more easily judge the birefringence of a mineral grain under observation by the dispersion caused by the standard thickness. On the other hand, many concrete petrographers prefer to work with much thinner sections. They have become accustomed to judging birefringence by comparison between mineral species.
When the size of the individual particles of interest is less than the thickness of the thin section,the particle boundaries tend to become obscured by the overlap of the particles. The finer the size of the particles, the more difficult it is to observe and define the boundaries. The less difference among the various optical properties of the particles (index of refraction, color, and birefringence), the more difficult it becomes to identify the boundaries and determine the identity of the particles.
Some of the aggregates whose structure must be determined accurately in concrete petrography are very fine-grained. The microstructure of the deleteriously reactive carbonate aggregates cannot usually be deciphered in thin sections of standard thicknesses. These rocks are composed of rhombic crystals of dolomite (5 to 20 µm across) "floating" in a murky calcite micrite. The murky appearance of the background is caused by submicroscopic particles of organic material, iron oxides, sulfides, and various other insoluble substances that are included in the micrite.When these materials include a relatively high percentage of opaque material, it can be impossible to discern details in a thin section as thick as 20 µm. The micrite itself may have a grain size of less than 5 µm.
The undulating extinction of alkali-reactive coarse-grained quartz can usually be seen in sections 25 to 30 µm in thickness; however, much of the reactive quartz present in metabasalts,
mylonites, and siltstones is exceedingly finemylonites, and siltstones is exceedingly fine—grained and cannot be detected in thin sections of standard thicknesses.grained and cannot be detected in thin sections of standard thicknesses.
Most cement is very fine. The greater portion will pass a No. 200 (75- µm) mesh sieve. The cement hydrates into even finer particles of low to negligible birefringence with nearly identical color and low indices of refraction. Observation of a thin section of HCC 25 µm in thickness could lead a rock—oriented petrographer to state that the material between the aggregate grains is groundmass. The term groundmass has long been used by geologic petrographers to indicate thatthe material is too fine for determinative microscopy. Examples of groundmass are the submicroscopic clay background of some sedimentary rocks and the material between phenocrysts in lavas or very fine-grained basalts. Thin sections with thicknesses on the order of 10 to 15 µm permit examination of the paste fraction, in which unhydrated portland cement grains, fly ash, and ground slag particles can be discerned. Much more detailed examinations ofthe HCC paste can be made using the SEM (see chapter 14).
Ideally, thin sections should be flat and cover a large portion of the glass on which they are mounted. Unless special equipment (not available at VTRC) is used, flaws, including lack of flatness and small areal extent, will sometimes have to be overlooked and the data will have to be collected from small thin sections that are thicker in the middle than at the edges.
Because these specimens are so thin, they must be mounted on glass microscope slides. Because of the thinness, the chance of lapping too far and losing the entire thin section is great. The procedure for examining thin sections consists of five steps:
| CAUTION: Examine the glass plate regularly for flatness. When it becomes dished because of the grinding action, discard and replace it. |
Because the most reactive textures in carbonate rock contain dolomite crystals that are very small (less than 25 µmacross), it is necessary to make the thin section used to discern the reactive texture thinner than the usual 25 to 30 µm so that the tiny rhombs of dolomite (often only half the thickness of a standard mineralogical thin section) can be seen without being obscured byother overlapping phases.
The thin section may be lapped down to zero thickness at one end and wedged toward a more standard thickness at the other (see figure 47). This may be accomplished by pressing more heavily on one end of the back of the section during the final finishing. By this method, there will exist at least a small area that is of the correct thickness to allow visibility of very small dolomite crystals. The pressure on the back of the section should be kept nearly evenly distributed until the section is down to about 25 µm in thickness.
The best thickness for viewing the texture of carbonate materials is usually achieved when observation with crossed polarizers indicates that much of the carbonate has begun to change colors;and it shows pink of the fourth order (or less) and the overall glaring is high -order white, generally typical of the carbonates. Experienced technicians may thin the entire section to the thickness best suited for viewing; however, the likelihood of loosing the entire section is very great.
The best compromise thickness for sections of concrete and some aggregates is about 10 µm. Each material studied will probably have its own optimum thickness. Unfortunately, the optimum is often quite thin and difficult to achieve without loss of the entire specimen cemented to the glass.
Figure 47. Thin section wedged to one end to observe dolomite rhombs in micrite as an indicator of possible alkali-carbonate reactivity (glass mount is 27 by 46 mm).

On occasion, the exact details of the texture and the aggregates of the wearing surface of an HCC pavement require examination and study with the petrographic microscope (Webb, 1970).
Cement two small portions of the surface under study to a slide with the wearing together in the center (to protect the area of interest from the greatest thinning action) produce a thin section of the thickness appropriate for the material and the purposesFigures 48 through 50 illustrate some of the steps in this process.
Sections for use with epifluorescent illumination (see chapter 13) are much like ordinary thin sections. The person fabricating the thin sections should become familiar with the material insection 5.3.1. The major difference is that the thin sections are impregnated with a fluorescent-dyed epoxy (Walker and Marshall, 1979; Wilk, et al., 1974; Roy, et al., 1993; St. John, et al., 1998; Jakobsen, Laugesen, and Thaulow, 2000). In these sections, the light emitted by the fluorescent dye is not collimated by the condenser because it is produced in the section closer to the objective lens than is the condenser. Therefore, the thinness of the section is of great importance in preventing the obscuring of the fluorescent details with fluorescent light from greater depths. Because the section is epoxy-impregnated and thus strengthened, it is not so difficult as might be thought to produce sections of 15 µm or less in thickness.
The methods used by VTRC to fabricate fluorescent thin sections (Walker and Marshall, 1979) were those that would involve the least cost for equipment. The Ingram-Ward thin-sectionequipment was already available, and the major purchases were the vacuum oven and the Syntron vibratory polishing lap.
It is necessary to make sure that the most impregnated side of the thin-section blank is very flat and that this side is cemented down to the final slide because the impregnation usually penetrates to a depth of only about 1.5 mm. With the Ingram-Ward equipment, a series of steps is necessary. Laboratories can easily devise satisfactory procedures for producing these sectionsusing the equipment available to them. Jakobsen, et al. (2000) provides a detailed description of their techniques.
Figure 48. Stage in the preparation of this section to show the details of the wearing surface: (A) Small blocks of wearing surface.
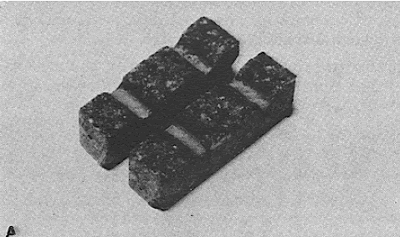
Figure 49. Stage in the preparation of this section to show the details of the wearing surface: (B) Blocks cemented to microscope slide.
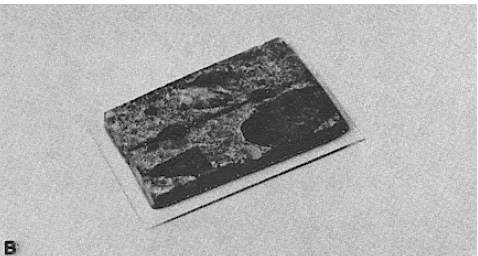
Figure 50. Stage in the preparation of this section to show the details of the wearing surface: (C) Thin section of blocks.
This section is about 50 µm in thickness because the study for which it was fabricated was concerned with the profile of the wearing surface and not with the identity and interrelationship of the component materials.
The fabrication of fluorescent thin sections is a 23-step process (as follows). When they are not in use, store specimens in a dark, airtight cabinet (to prevent fading of the fluorescence). To prevent carbonation, use an effective desiccant (e.g., Drierite®) and a carbon dioxide absorbant (e.g., Ascarite®).
| WARNING: Persons unfamiliar with the hazards of these compounds are referred to the Chemical Safety Data Sheets published by the Manufacturing Chemists Association, Inc., 1825 Connecticut Avenue, NW, Washington, DC 20009, or to the Materials Safety Data Sheet that should be obtained from the supplier of the compound. |
| CAUTION: Examine the glass plate regularly for flatness. When it becomes dished because of the grinding action, discard and replace it. |
| WARNING: Do not immerse any portion of your hand in an ultrasonic bath while the ultrasound is on. The cavitation caused by the ultrasound can erode fingernails and damage skin. |
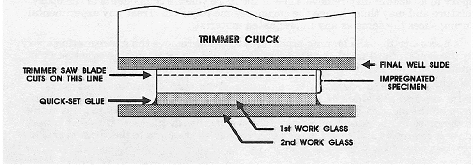
Figure 51. Specimen mounted between work glasses and final slide (dotted line indicates the plane the saw will cut).
Small particles of rock, paste, reaction products, and sand grains can be identified and examined with the petrographic microscope; however, they cannot be examined unmounted. The relief between the index of refraction of the substance and the index of refraction of air is so great that the internal reflection within the particle prohibits observation of any useful properties. Therefore, it is necessary to prepare mounts in which these particles are submerged in a transparent substance with an appropriate refractive index. Such mounts are called grain mounts. Temporary grain mounts are usually made with refractive index (immersion) oil for the purpose of determining the index of refraction and other optical properties of small particles of material. The oil can easily run off the mount and, in time, will evaporate. Permanent grain mounts are made with epoxy and can be kept indefinitely for demonstration and archival purposes.Permanent grain mounts can be thinned in the Ingram-Ward equipment, as are thin sections (see section 5.3.1), to increase the transparency of the particles and facilitate the observation of many of the optical properties of the material.
Grain mounts are more satisfactory if the particles in any one mount are nearly the same size.Use the 76-mm sieves to separate the sizes. If the examination is concerned with the relative amounts of the sizes, weigh each sieve fraction.
This procedure is a five-step process:
This procedure is a one-step process: Fabricate a permanent grain mount with epoxy, Canada balsam, or a similar substance of a known refractive index. Such mounting materials may be used purely to preserve the specimen or to provide a secure base, so that once the mounting material is properly cured, the preparation can be thinned and finely lapped as a thin section of the material.
the petrographer in order to determine its suitability for use as aggregate in HCC. Specimens may be submitted as part of ongoing research concerning some form of HCC deterioration or forcomparison with the aggregate found in HCC specimens presently undergoing investigation. The aggregate specimens can be best examined and tested if they are properly prepared for the proposed examination and testing (see ASTM D 75).
Particulate materials can become segregated into sizes or into mineralogical portions when they are stockpiled or even when they are shoveled or poured into small piles. The ability of the particles to move over each other and the substrate is affected by the density, geometry, shape, and surface texture of the particles (Mullen, 1978). Air resistance may affect the placement movement of very fine sizes of materials. Each type of material has its own characteristics that affect its manner of movement, thus allowing both vertical and horizontal segregation within a pile of particles.
This procedure is a four-step process:
This procedure is a three-step process: