 |
Manual of Practice for an
|
LIST OF FIGURES
1. Outline of the components of an anti-icing program in the context of a winter maintenance program.
2. Hopper type spreader.
3. Under-tailgate spreader with prewetting equipment.
4. Simple roof over stockpile.
5. Enclosed structure for chemical storage.
6. Single disk liquid spreader.
7. Liquid spreader using a distributor bar with nozzles.
8. Chassis-mounted liquid spreader.
9. Slip-in liquid spreader.
10. Tow-behind liquid spreader.
11. Kansas DOT salt brine production facility.
12. Testing of salt brine concentration with a hydrometer.
13. Outside CMA mixing facility operated by Washington DOT.
14. Overhead spraybar for prewetting chemical load in truck.
15. RWIS roadside installation.
16. Phase diagrams of NaCl and CaCl2 solutions.
17. Phase diagrams of five chemical solutions.
LIST OF TABLES
1. Pure salt concentration and corresponding specific gravity (measured by a hydrometer) at 15°C (59°F).
2. Calcium chloride mixing proportions.
3. Gradation of salt specified by ASTM D 632. 43
4. Salt gradation specified by British Standard BS 3247 Specification for salt for spreading in highways for winter maintenance.
5. Swedish gradation for salt.
6. Finnish gradation for salt.
7. Proportions for preparing sodium chloride solutions from commercial grade salt (i.e., up to 5 percent impurities).
8. Weather event: light snow storm.
9. Weather event: light snow storm with period(s) of moderate or heavy snow.
10. Weather event: moderate or heavy snow storm.
11. Weather event: frost or black ice.
12. Weather event: freezing rain storm.
13. Weather event: sleet storm. 62
Technical Report Documentation Page
FHWA-RD-95-202 |
|||
|
MANUAL OF PRACTICE FOR AN EFFECTIVE ANTI-ICING PROGRAM A Guide For Highway Winter Maintenance Personnel |
June 1996 |
||
|
|
|||
|
Stephen A. Ketcham, L. David Minsk, Robert R. Blackburn, Edward J. Fleege |
|
||
|
US Army Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, New Hampshire 03755-1290 |
3E6B |
||
|
DTFH61-93-Y-00123 |
|||
|
Office of Engineering Research & Development Federal Highway Administration Turner-Fairbank Highway Research Center 6300 Georgetown Pike McLean, Virginia 22101-2296 |
Manual of Practice June 1993 - June 1995 |
||
|
|
|||
|
Contracting Officer’s Technical Representative (COTR) - Brian Chollar (HNR-20) The offices of Engineering, Engineering R&D and Technology Applications sponsored and managed this project as part of the TE 28 "Anti-icing Technology" Program. |
|||
|
Highway anti-icing is the snow and ice control practice of preventing the formation or development of bonded snow and ice by timely applications of a chemical freezing-point depressant. It provides a maintenance manager with two major capabilities: the capability for maintaining roads in the best conditions possible during a winter storm, and the capability to do so in an efficient manner. As a consequence, anti-icing has the potential to provide the benefit of increased traffic safety at the lowest cost. However, to achieve this benefit the maintenance manager must adopt a systematic approach to snow and ice control and must ensure that the performance of the operations is consistent with the objective of preventing the formation or development of bonded snow and ice. Such an approach requires use of considerable judgment in making decisions, requires that available information sources be utilized methodically, and requires that the operations be anticipatory or prompt in nature. This manual provides information for successful implementation of an effective highway anti-icing program. It is written to guide the maintenance manager in developing a systematic and efficient practice for maintaining roads in the best conditions possible during a winter storm. It describes the significant factors that should be understood and must be addressed in an anti-icing program, with the recognition that the development of the program must be based on the specific needs of the site or region within its reach. The manual includes recommendations for anti-icing practices and guidance for conducting anti-icing operations during specific precipitation and weather events. |
|||
|
Highways, anti-icing, snow and ice control, preventive strategy, bonded snow and ice, application timing, chemical freezing point depressant, deicing |
No restrictions. This document is availible to the public through the National Technical Information Service, Springfield, Virginia 22161 |
||
Unclassified |
Unclassified |
70 |
|
Application of a chemical freezing-point depressant onto a highway pavement at the start of a winter storm, or even prior to the beginning of precipitation, inhibits the development of a bond between the snow or ice and the pavement surface. Furthermore, moderate and periodic reapplications of the chemical during the storm can continue this effect. Such preventive operations are the core of an anti-icing program.
Anti-icing is the snow and ice control practice of preventing the formation or development of bonded snow and ice by timely applications of a chemical freezing-point depressant. It provides a maintenance manager with two major capabilities: the capability for maintaining roads in the best conditions possible during a winter storm, and the capability to do so in an efficient manner. As a consequence, anti-icing has the potential to provide the benefit of increased traffic safety at the lowest cost. However, to achieve this benefit the maintenance manager must adopt a systematic approach to snow and ice control and must ensure that the performance of the operations is consistent with the objective of preventing the formation or development of bonded snow and ice. Such an approach requires use of considerable judgment in making decisions, requires that available information sources be utilized methodically, and requires that the operations be anticipatory or prompt in nature.
In contrast to anti-icing operations, a common procedure of traditional snow and ice control practice is to wait until an inch or more of snow accumulates on the pavement before beginning to plow and treat the highway with chemicals or abrasives. While this procedure is straight-forward, it frequently leads to a compacted snow layer (pack) that is tightly bonded to the pavement surface. A subsequent "deicing" of the pavement is then necessary, usually requiring a large quantity of chemical to work its way through the pack to reach the snow/pavement interface and destroy or weaken the bond. Because this operation is reactionary, it requires less judgment than anti-icing. Yet as a result of its inherent delay, it often provides less safety, at higher cost, than anti-icing. Nonetheless the reactive technique of deicing will remain important for snow and ice control, as there will always be lower priority service levels that preclude preventive operations.
Anti-icing is well suited to routes with a higher level of service. This is because the vigilance and timeliness of successful anti-icing operations are most compatible with service levels requiring earlier and higher frequency winter maintenance operations. It is also because the preventive nature of anti-icing can support higher service level objectives such as maintaining bare pavement throughout a storm or returning to bare pavement as soon as possible following pack formation. In fact, because of the demanding requirements of higher service levels, many maintenance forces in the United States have been instinctively implementing elements of anti-icing practices for years. Sufficient evidence has accumulated from 2 years of Strategic Highway Research Program (SHRP) and 2 years of Federal Highway Administration (FHWA) anti-icing testing to demonstrate the effectiveness of US anti-icing practices, which has culminated in this manual.
1.1 PURPOSE OF THE MANUAL
This manual provides information for successful implementation of an effective anti-icing program. It is intended for use by highway agency maintenance managers at headquarters and subareas as well as field personnel.
The manual is written to guide the maintenance manager in developing a systematic and efficient practice for maintaining roads in the best conditions possible during a winter storm. It describes the significant factors that should be understood and must be addressed in an anti-icing program, with the recognition that the development of the program must be based on the specific needs of the site or region within its reach. It focuses on the weather information, materials, and methods that will best address site conditions such as level of service, highway agency resources, climatological conditions, and traffic.
1.2 SCOPE AND ORGANIZATION OF THE MANUAL
Following the Introduction, this manual is divided in three sections plus appendixes:
Section 2 presents an introduction to anti-icing in the context of an agency’s winter maintenance program.
Section 3 describes the operational, decision making, and personnel management capabilities needed to support an anti-icing program. It contains three major components: operations toolbox, decision-making toolbox, and personnel toolbox.
Section 4 describes the operations and practices of anti-icing. It includes recommendations for successful anti-icing practices that can be employed for various combinations of precipitation, temperature, traffic volumes, and mandated levels of service. It complements the information provided in section 3, and serves as a companion and background document for the specific and concise guidance in Appendix C. The Section contains four major components: initial operations, subsequent operations, special considerations, and post-storm assessment of operations. It and the guidance of Appendix C are based on analysis of data obtained during the winters of 1993/94 and 1994/95 in the 15 States participating in the FHWA Test and Evaluation Project 28 (T&E 28) (1) [ Underlined numbers in parentheses refer to references at the end of this document.] , and also on the relevant experience and review of anti-icing practices from SHRP Project H-208 (2).
Supporting and auxiliary material regarding chemicals is presented in the Appendixes A and B. Appendix C presents guidance for conducting anti-icing operations during specific precipitation and weather events.
A winter maintenance program consists of several elements with varying degrees of importance depending on the size of the operational jurisdiction it covers and the complexity of its road network. One element, level of service (LOS), is important for all jurisdictions. It must be considered, along with the climatic conditions, in the design of any snow and ice control program. This Section describes how the level of service and climatic conditions influence an anti-icing program, and how anti-icing fits into the context of an overall winter maintenance program and level of service.
2.1 LEVEL OF SERVICE
The extent to which maintenance services will be provided to a road section is determined by management by the assignment of a level of service. In the case of winter maintenance, this will require establishing a prescribed end-of-storm road condition, what intermediate conditions will be acceptable while obtaining that condition, or the frequency of snow and ice control maintenance operations. Some examples of LOS are: maintain 24-h coverage until near normal surface conditions are restored with coverage at a rate of 2 to 4 cycles (passes one vehicle would make over a given point on the road) per shift (however defined) as conditions and resources allow; bare pavement during daylight hours; center bare at all times; or snow-covered to a maximum of 12 mm (1/2 in) during daylight hours. The LOS will largely be determined by the importance of the road, and hence the average daily traffic. As a reflection of the desired bare pavement condition, high winter maintenance service levels are often generically called "bare pavement policies."
As defined in the Introduction, anti-icing is the snow and ice control practice of preventing the formation or development of bonded snow and ice by timely applications of a chemical freezing-point depressant. A winter maintenance crew that is persistent in this practice is best able to support demanding road condition requirements set forth by a higher LOS. If the LOS requirements are in terms of operational frequency rather than road condition, a crew operating at higher frequency would find anti-icing practices to provide the best road conditions possible within a given set of operational constraints. Because of the proven compatibility between anti-icing and higher LOS, this manual presents anti-icing as a strategy for support of high service levels.
Figure 1 depicts the components of an anti-icing program in the context of a winter maintenance program and the LOS assignment. It shows anti-icing as a support strategy for "bare pavement" service levels.
2.2 CLIMATIC CONDITIONS
In addition to the service level, maintenance effort will vary with climatic conditions. A factor of great importance is pavement temperature. Pavement temperature directly influences the formation, development, and breaking of a bond between fallen or compacted precipitation and the road surface as well as the effectiveness of chemical treatments. It is also important when high humidity levels are accompanied by low dew point temperatures. Under these conditions there will be a greater potential for formation of frost and black ice. Unless some external source of heat is provided, the pavement temperature will generally track air temperature with a time delay. For road sections without obstructions to a clear sky view, solar radiation during the day and exposure to the clear night sky will affect the road surface temperature to a greater extent than on sections influenced by air contact only.
Other important climatic factors are type and rate of precipitation. Together with pavement temperature, they are the most important variables to consider when performing anti-icing operations. The operational guidance described later in this manual is presented in terms of these variables.
2.3 DEICING AND ANTI-ICING
There are two distinct snow and ice control strategies that make use of chemical freezing-point depressants: deicing and anti-icing. They differ in their fundamental objective. Whereas anti-icing operations are conducted to prevent the formation or development of bonded snow and ice for easy removal, deicing operations are performed to break the bond of already-bonded snow and ice. Deicing is familiar to most agencies since it has been the most widely used strategy in the past. The design of deicing operations as a bond-breaking operation stems from its timing: it is commonly initiated only after 25 mm (1 in) or more of snow has accumulated and bonded to the road.
A distinction between anti-icing and deicing has not always been clear. This is because anti-icing is a relatively new term. Some agencies that have instinctively adopted anti-icing practices over the years have used the term deicing to describe their operations, even when they were intended to prevent a strong ice-pavement bond. In this manual a distinction between a primarily-preventive and a primarily-reactive strategy is intended when referring to anti-icing and deicing, respectively.
There are other strategies and techniques that can and have been used in snow and ice control operations. These include such approaches to a bare pavement policy as use of heated pavements, structural covering of a road to protect it from precipitation (e.g., covered bridges or avalanche sheds), and the use of a modified pavement surface in conjunction with plowing, to name a few. These are not covered in this manual.
2.4 ANTI-ICING AS A SYSTEMATIC PRACTICE
As illustrated in Figure 1, both deicing and anti-icing can be used to support higher service level objectives. However, because deicing is reactionary, it cannot support strict requirements for safe road conditions during a winter storm. Anti-icing can meet such requirements, but to successfully and efficiently do so the maintenance manager must ensure that the timing of the operations is consistent with the objective of preventing the formation or development of bonded snow and ice. This is not an easy task. It requires use of much more judgment in making decisions, requires that available information sources be utilized methodically, and requires that the operations be anticipatory or prompt in nature. In short it requires a systematic approach.
The elements of a systematic anti-icing program are shown in Figure 1. As indicated, support of an anti-icing strategy is divided into tools and operations. The supporting tools can be organized according to operations, decision-making, and personnel "toolboxes," which are further broken down according to capabilities, information sources, and procedures that may be available for a given operation. A toolbox analogy is followed to suggest that managers should use their available resources systematically as they would use mechanical tools in the course of a methodical repair job. Operations are broken down into initial and subsequent operations in order to convey the importance of the initial chemical treatment in anti-icing operations, and to signal that subsequent operations throughout a storm or weather event should follow the anti-icing strategy as well.
While the outline of Figure 1 indicates the underlying complexity of anti-icing practices, it also reveals that what may initially be thought of as an overly demanding practice is actually an organized set of preparations, decisions, and operations. Such a methodical program can be designed for an agency’s unique conditions using tools that are generally available to agencies today and using operational guidance provided later in this manual. The tools are described in Section 3, and the operational guidance is presented in Section 4 and Appendix C.
2.5 ABRASIVES USE
Common to many snow and ice control operations is the use of abrasives. It is recognized that abrasives may be necessary when a rapid increase in friction coefficient is required, particularly at temperatures so low that chemical action is slow, and in conditions where snow or ice is strongly bonded to the pavement and cannot easily be removed. As these latter conditions are more likely to occur in the course of deicing, abrasives treatments can be an important tool for deicing operations. Abrasives are not ice-control chemicals, however, and will not support the fundamental objective of either anti-icing or deicing. Their sole function is to increase the coefficient of friction. This increase may be short lived, because traffic will rapidly disperse abrasives.
There is generally no advantage gained from the routine use of abrasives in an anti-icing program. When anti-icing operations have successfully prevented or mitigated the hazards of packed snow, for example, straight abrasives applications will provide no significant increase in friction or improvement in pavement condition. Further, a mix of abrasives and chemical will usually be no more effective as an anti-icing treatment during snowstorms than the same amount of chemical placed alone. It even appears that the use of abrasives in the mix can be detrimental to the effectiveness of the chemical. Because of the cost associated with both application and clean-up of roads and drainage facilities, and because of the potential airborne dust problem accompanying their use, abrasives applications should not be a routine operation of an anti-icing program.
Although this manual does not present abrasives application capability as a tool for anti-icing, Section 4 and Appendix C do provide guidance for conditions under which abrasives use may be appropriate during operations. Recommended practice for abrasives applications, or for deicing, is not provided in this manual.
As indicated in Figure 1, the supporting tools of an anti-icing strategy are organized into an operations toolbox, a decision-making toolbox, and a personnel toolbox. Each comprises a combination of new, conventional, and traditional technologies, and contains capabilities, information sources, or procedures that address the toolbox function.
The operations toolbox includes capabilities for applying solid chemicals, "liquid" chemicals (i.e., chemical solutions), or prewetted solid chemicals, and for plowing. The decision-making toolbox includes long- and mid-term weather forecasts, road and road weather information, nowcasting, traffic information, patrols providing information on weather and pavement conditions, and evaluations of treatment effectiveness. The personnel toolbox consists of personnel trained in anti-icing practices and use of information sources for decision making, and stand-by and call-out procedures.
In the development of an anti-icing program, each toolbox should be viewed as a critical component of a systematic operation or practice. The required elements of the toolboxes will differ from site to site, jurisdiction to jurisdiction, or agency to agency, depending primarily on levels of service, highway agency resources, and climatic conditions, and so the selection of the tools will differ in each program design. In addition, as newer technologies become available, as more effective operational techniques are identified, and as a program is accordingly developed over the course of time, the toolboxes will expand and their elements improve. However, it will always be important that the maintenance manager select and maintain effective anti-icing support tools in the subject areas of the outline: operations, decision making, and personnel. The discussion below is written as an initial guide to assist the maintenance manager in this process.
3.1 OPERATIONS TOOLBOX
This discussion of the operations toolbox is divided into four major categories: solid chemical application capability, chemical solution application capability, prewetted solid chemical application capability, and plowing capability. Each of these is described below. Material and equipment requirements, and discussions of techniques, are included in the descriptions.
3.1.1 Solid chemical application capability
The use of dry solid chemicals as an anti-icing treatment can be effective in many circumstances, but only those where there is sufficient moisture or accumulation on the pavement. Moisture must be available for two reasons: to prevent loss of material off a dry pavement, and to trigger the solution of the salt. For initial operations, solid chemicals will be effective when a maintenance team has the operational resources to apply the chemical as soon as possible after sufficient precipitation has fallen, but before snowpack or ice bonds to the pavement. For subsequent operations, solid chemical treatments will usually be effective as there is typically adequate moisture or accumulation during later periods of storms. Nonetheless, for either initial or subsequent operations, when there is not enough moisture or accumulation on the pavement there is likely to be loss of the chemical from the pavement. This may be caused by the blowing action of traffic, especially from high speed and commercial vehicles, or by the bouncing of particles off the pavement during spreading. It is not uncommon to see dry solid chemicals rebound up to 1/2 m (2 ft) from a dry pavement after being distributed from a conventional spreader spinner, although recently-introduced zero-velocity spreaders have enabled the placement of solid chemicals on the pavement with minimum bounce.
The material and equipment requirements for solid chemical applications are similar or identical to those used conventionally or well known by most agencies. These are discussed here.
3.1.1.1 Solid materials and gradation
The solid chemical most commonly used for anti-icing treatments is salt, or sodium chloride. A mix of solid sodium chloride and solid calcium chloride has been used by some agencies, and in some instances straight calcium chloride has been used. In fact, almost any solid chemical that has been used for deicing also can be used for anti-icing depending on operating conditions. Information on solid chemicals and solubility can be found in Appendixes A and B.
The gradations of salt used for anti-icing treatments have mostly been particle size distributions designed for deicing operations, which is appropriate in the absence of prewetting. A discussion of gradation is presented in Appendix A.
Solid material spreaders
Originally salt was spread during snow and ice control operations by shoveling it onto the road from the truck bed. Control of application rate was not possible, and the physical exertion required over long stretches of the road was demanding.
Later, salt spreaders which could be attached to a truck were developed along the line of the fertilizer distributors used in agriculture. This was the beginning of attempts to achieve a more uniform method of applying salt to the road. Rate control of the material, however, was still not satisfactory because the spreading rate was not varied as a function of truck speed. Over-spreading occurred when the truck came to a stop, and under-spreading when the truck accelerated. When the economic and environmental demands became evident, manufacturers of spreading equipment modified their equipment to distribute snow and ice control materials at controlled flow rates and at speeds higher than were used for agricultural purposes.
Nowadays dry chemicals are applied to the roadway by means of either a hopper type spreader (Figure 2) or a dump body with an under-tailgate spreader (Figure 3). These spreaders are capable of spreading free-flowing granular materials from a minimum width of 1 m (3 ft) to a maximum of 12 m (40 ft). Generally the hopper spreaders are self-contained units mounted in dump-trucks in winter, then removed and stored in other seasons so that the trucks may be used for other maintenance work. These units consist of a steel V-box body, discharge/feed conveyer, spinner disc, power drive, and other necessary components. The V-box spreaders have hopper capacities of 3.4 to 13.2 m3 (4.5 to 17.2 yd3). At the hopper’s base is a full-length feed system whose speed is controlled from the truck cab. This feed system can be either a full-length belt, chain-drag belt, or a longitudinal auger. These systems feed material into a chute where it falls onto a spinner that spreads it laterally across the road.
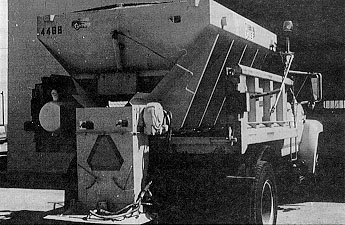
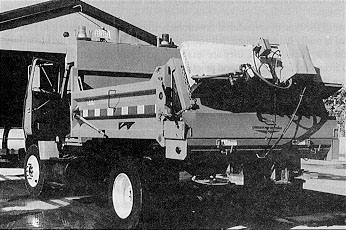
The under-tailgate spreader is also a self-contained unit that slips into a dump body and is easily removed and hooked up. These devices consist of a small hopper, an auger feed mechanism, hydraulic driver system, and a spinner disc. Both under-tailgate and hopper spreaders have been used successfully for anti-icing operations.
The variables affecting application rate of a given material are 1) area of the gate opening on a hopper box or the opening in the bottom of the tailgate hopper, 2) feed-belt or auger speed, and 3) truck speed. The gate opening height is an adjustment made at the time of calibration and generally is not changed during spreading operations. Thus, to control the actual spreading rate, the speed of the feed belt or auger needs to be considered along with truck speed. The methods that can be used to control the spreading rate of spreaders fall into three categories: 1) no control, 2) manual control, and 3) automatic control.
Automatic control of material application rates is achieved with ground-speed-oriented controllers. These units automatically adjust a pressure-compensation valve. A truck operator with an automatic controller is able to maintain a constant application rate of material on the road without having to adjust the valve opening to conform to the changing speed of the truck. To spread a constant amount of material along a road, a truck operator need only select an application rate. With some controllers, the spreading width can also be selected. Changes can be made at any time during operation - the automatic controller does the rest. Manual control, however, requires continual adjustment of the valve to maintain a constant application rate.
Automatic controllers use a truck-speed sensor for adjusting the opening of the pressure-compensation valve which in turn controls the operating speed of the feed mechanism. Various types of truck-speed sensors are available. Most are connected to the speedometer cable, while some measure the rotation of the drive shaft or a wheel. There is not enough information in the literature to suggest which type of sensor is the most durable, reliable, or appropriate for anti-icing operations. However, all commercially available units appear to be equally suitable.
There are two types of automatic controllers: the open-loop system and the closed-loop system. Both types require a speed sensor. The open-loop controller uses only this sensor to adjust the hydraulic valve opening. In the closed-loop system, a second sensor detects the operating speed of the feed-belt or auger. The closed-loop controller integrates the two signals to adjust the hydraulic valve opening automatically.
Many maintenance managers believe that an open-loop controller with its single truck-speed sensor provides adequate control of the application rate. They feel that annual equipment maintenance and calibration avoid the need for the second sensor provided with the closed-loop system. Some also believe that, because much material is not uniform but lumpy, very close control of the feed-belt or auger speed is not necessary.
Other users prefer the multiple control provided by closed-loop controller systems. They feel the second sensor is needed to correct changes which occur during snow and ice control operations such as wear of spreader equipment and variations in performance of the spreader’s hydraulic fluid. Wear can change the calibration of the equipment. Also, the variable operating temperature and aging of the spreader’s hydraulic fluid change the operation of the feed-belt, auger and the spinner motors.
Regardless of the type of spreader, it is extremely important to calibrate it to ensure that the desired quantity of material is really being applied. All equipment should be calibrated before winter operations begin. Though most agencies do this, they are less likely to check the manufacturer’s calibration on new equipment delivered from the factory. It is good practice to do so. Also, many agencies assume calibration is necessary only before the season begins and don’t realize that settings may change with use. Changes in mechanical linkages and components may occur, and hydraulic systems perform differently as the season progresses. It is also good practice to recalibrate the spreader equipment after any maintenance is performed on the spreader/truck system.
Anti-icing operations generally require applying a controlled quantity of chemical (often as little as 30 kg/lane-km (100 lb/lane-mi), and in some cases even less) uniformly across the road. To determine a spreader’s capability to control application rate and the distribution pattern, the equipment can be evaluated and calibrated following the "Proposed American Testing Protocol for Winter Maintenance Spreaders." This protocol is reported in SHRP H-385 report Development of Anti-Icing Technology (2). Another procedure for calibrating application rate is outlined in the Salt Institute’s The Snowfighter’s Handbook (3).
Solid chemical storage facilities
Solid chemicals should be stored under cover or inside a building. Chemicals stored in the open pick up moisture, produce leachate which drains into water sources, and develop a waste outer crust. When loaded into a spreader, the crust breaks into lumps that may clog the spreading equipment or will at least interrupt the feeding of the spinner.
Many types of barns or silos are in use for chemical storage, ranging from a simple roof over the stockpile (Figure 4) to a complete building (Figure 5). These facilities may also house the spreader(s) under the same or appended roof.
The number of storage loading points for a given roadway system is determined by the following considerations: 1) the maximum cycle time allowed for a spreading operation; 2) the level of service of the road segments to be treated; 3) special features such as bridges, tunnels, and intersections; and 4) the route length of the spreaders. The route length determines the number of material storage locations (sheds) and the way they are distributed geographically. It is possible that the route length or range of operation of a spreader used in anti-icing operations can be expanded over that used for conventional practices. As such, the important factors in determining the number of storage locations are the first three enumerated above. It is possible that the number of storage loading points can be consolidated through the use of anti-icing operations.
3.1.2 Liquid chemical (chemical solution) application capability
Though solid or prewetted solid chemicals can be used as anti-icing treatments, there are advantages to use of liquids in small amounts for some conditions at pavement temperatures of about -5oC (23oF) and above. These include the ability to place chemical uniformly over the pavement at relatively fast spreading speeds, and the ability to place chemical onto dry pavement as a pre-storm treatment to avert delays that may lead to bonded snow or ice. However, this means putting the chemical down before enough snow has accumulated to keep the chemical from reaching the pavement or from being excessively diluted. In some situations it may be beneficial to remove snow and slush from the road using methods that are more thorough than conventional techniques. This will be discussed later in Section 3.1.4 on plowing. Liquids can be used at pavement temperatures below -5oC (23oF) by increasing the application rate over the levels recommended for -5oC (23oF) and above. The cost effectiveness of using higher liquid chemical application rates at lower pavement temperatures needs to be evaluated on a case-by-case basis.
3.1.2.1 Chemical solutions
Five chemicals have been used for liquid anti-icing treatments: sodium chloride (NaCl), magnesium chloride (MgCl2), calcium chloride (CaCl2), calcium magnesium acetate (CMA), and potassium acetate (KAc). Appendix A presents information on the properties of these chemicals and instructions for preparing various liquid concentrations. Appendix B contains information on the freezing-point of these five brines as a function of the solution concentrations.
Liquid application equipment
There are two principal types of liquid application equipment for highway use. One uses spinners consisting of either multiple rotating disks or a single disk (Figure 6). The other type uses nozzles on a distributor bar (Figure 7). Either spreader may be chassis-mounted (fixed on the frame) (Figure 8); be a "slip-in" unit that can be placed temporarily in the bed of a dump truck or on the frame and removed during the off-season (Figure 9); or it can be a trailer, or "tow-behind" unit (Figure 10).

Figure 6. Single disk liquid spreader.

Figure 7. Liquid spreader using a distributor bar with nozzles.
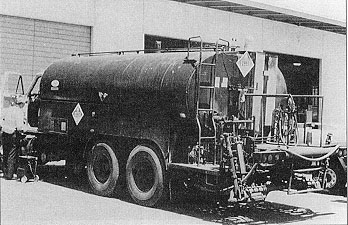
Figure 8. Chassis-mounted liquid spreader.
Until recently, most liquid applicators were made in Europe and Scandinavia. Several United States firms now provide highway spreader equipment and spreader components for distributing liquid chemicals (and prewetted solids).
Descriptions of the capabilities of a nozzle-type and a spinner-type spreader are presented here. They are provided only as examples for discussion purposes, and are not to be taken as endorsements or as recommended specifications.
Nozzle-type spreader. This spreader, shown in Figure 10, sprays the liquid from nozzles at a low height above the road to reduce the influence of air turbulence behind the vehicle that can cause the liquid to disperse before hitting the pavement. The unit is designed to be towed by a truck equipped with a liquid tank. The spreader is powered by its own traction-driven wheel and also has a ground-speed control.
In this particular unit, the liquid chemical flows by gravity through a clear 75 mm (3 in) plastic hose from the tank to the spreader, where two liquid pumps provide an adequate supply of liquid chemical to an array of nozzles. The two diaphragm pumps share a common inlet filter. The spraybar is 2.3 m (89 in) long and is equipped with six nozzles--three large and three small. The large nozzles spray over a 3.5 m (11.5 ft) width. At low speeds, the smaller nozzles are used. As the truck’s speed increases and the need for liquid volume increases, the smaller nozzles are shut off automatically and the larger nozzles are supplied without a break in spray pattern. As the truck slows down, the reverse process occurs.
Three additional stainless steel nozzles are mounted on the left end of the spray bar. These apply liquid to the left lane. A total width of 7 m (23 ft) can be sprayed with the spraybar and side nozzles. Each side nozzle is pressure regulated to ensure an even flow at all speeds.
Spinner-type spreader. This unit, shown in Figure 6, attaches to the rear of a truck equipped with liquid tanks. The unit can be powered either by the vehicle’s hydraulic system or by a separate road wheel.
In this unit, the liquid chemical is pumped by two impeller pumps to a specially designed stainless steel spinner. This is slightly convex with 10 curved vanes, and the spinner mechanism is manually adjustable to give asymmetric or symmetric spread patterns. The unit can apply liquid over a spread width of 2 to 8 m (7 to 26 ft) while traveling between 10 and 60 km/h (5 and 40 mph).
There is not enough information in the literature to suggest which type of liquid spreader is the most reliable or effective for anti-icing operations. The nozzle type can have problems with nozzles plugging or can dispense such a fine mist that it disperses before hitting the pavement surface. Some nozzle type designs incorporate large size (6 mm (0.25 in) or larger) spray nozzles that increase the reliability of spray equipment because they require less liquid filtration and are less likely to clog. Spinner disks can dispense liquid droplets that are too large for uniform coating of the pavement surface. A highway agency interested in using a liquid spreader for anti-icing operations needs to review the spreader manufacturer’s claims carefully.
Highway agencies interested in beginning or experimenting with an anti-icing program might consider modifying some existing spreader equipment before investing in new equipment. Asphalt distributor trucks, liquid fertilizer spreaders, and spreaders used to spray for weed control have been modified and successfully used by some highway agencies (Nevada Department of Transportation and Colorado Department of Transportation to name only two) in their initial anti-icing programs. California Department of Transportation (Caltrans) has built a customized spray bar that is capable of applying 100 L (25 gal) of solution per lane mile at speeds up to 50 km/h (30 mph). Based on just 1 year’s positive experience with the spray bar, Caltrans decided to build two additional liquid spreaders.
Regardless of the type of liquid spreader used, it is extremely important to calibrate it to ensure that the desired quantity of material is actually being applied. All equipment should be calibrated before winter operations begin. Though most agencies do this, they are less likely to check the manufacturer’s calibration on new equipment delivered from the factory. It is a good practice to perform this calibration. Also, many agencies assume calibration is necessary only before the season begins and don’t realize that settings may change with use. Changes in mechanical linkages and components may occur, and hydraulic systems perform differently as the season progresses. As a check, compare the controller application rate setting against the volume remaining in the tank several times during the winter for any deviation. It is a simple matter with most liquid applicators to use a dipstick for a check.
An application that leaves the surface merely damp will be sufficient in many conditions. Since these applications do not result in flow of liquid on the pavement, the uniformity of spread must be achieved at time of application. If a nozzle or filter is plugged, that part of the road the spray normally "sees" will remain untreated. Nozzles and filters need to be checked frequently.
Spreader speed must be evaluated for the particular conditions. Turbulence will affect spread coverage uniformity. It would be desirable to have a spreader that could perform adequately at speeds close to those of the traveling public in order to reduce the speed differential and improve the safety of the operation.
Generally, experience has shown that liquid chemicals can be successfully applied at speeds up to 40 to 55 km/h (25 to 35 mph) for spinner type spreaders and at speeds up to 65 to 80 km/h (40 to 50 mph) for spray bar type spreaders. Turbulence behind the spreader truck prevents a uniform distribution pattern at higher application speeds.
The liquid supply tanks used on spreader vehicles should be made of non-corrosive material such as polyethylene. Some States have used stainless steel tanks but this adds to the weight of the system. A number of highway agencies have installed 11 m3 (3000 gal) truck-mounted tanks for use on the spreaders. They soon discovered, due to weight limitations and density of the liquid chemicals used, only 7.5 m3 (2000 gal) of liquid could be carried on the standard size truck. Also, they experienced problems with the liquid sloshing in a tank without internal baffles. It is recommended that truck-mounted tanks larger than 5.5 m3 (1500 gal) be equipped with internal baffles.
Chemical solution production facilities; salt brine
Simple NaCl brine manufacturing plants that can operate relatively trouble-free became a necessity with the use of salt brine or prewetted salt for anti-icing treatments. Highway agencies working with private companies have designed a number of salt brine production plants. As a result, there are several companies that manufacture brine production systems.
Two types of manufacturing plants are currently in use for preparation of saturated brine: batch and continuous flow. Simple batch units for temporary or small scale production can be assembled using small tanks. Water passed through a bed of rock salt by gravity will produce a solution saturated at the water temperature. Production involves loading a tank with salt and running water through it, collecting the brine in a holding tank. From there the brine is passed through a 10 µm filter or pumped into a storage tank or directly into a spreader truck. Production rates are somewhat low with this process, about 8 L/s (600 gal/h). Several agencies have assembled their own simple plants like this. The plant used by Kansas DOT is shown in Figure 11.
The concentration should be checked during production with a hydrometer (Figure 12). This device measures the specific gravity of the solution which will increase as the concentration increases. Table 1 lists hydrometer readings and the corresponding salt concentration for a solution temperature of 15°C (59°F). More efficient continuous flow units have been developed for high capacity production. These plants force water under pressure through a bed of salt. The saturated solution then flows into a storage receptacle. Salt and water are metered automatically and continuously.

Figure 12. Testing of salt brine concentration with a hydrometer.
The following items should be considered in specifying or designing a brine manufacturing plant:
Future needs for additional capacity
Adequate water inlet capacity
Suitability of the proposed site from an operational and environmental standpoint
Pump capacity requirement
Possibility of using earth heat for storage tanks
Overflow control requirement
Containment of spills
Use of noncorrosive material in the plant construction
Chemical solution production facilities; liquid CMA
A CMA solution is prepared by dissolving solid CMA in water, resulting in a murky solution that settles over time to produce a clear CMA solution on top and insoluble waste on the bottom. It is recommended that the solution concentration not exceed about 25 percent. The solution tends to be stable at this concentration. If the concentration exceeds 28.5 percent, the dissolved CMA will tend to recrystallize which will clog the spray nozzles on the liquid spreaders. The recommended 25 percent concentration is prepared by mixing 1.36 kg (3.00 lb) of CMA per 3.78 L (1 gal) water. For example, 1000 kg (2205 lb) of CMA mixed with 2.78 m3 (735 gal) of water will produce 3.31 m3 (875 gal) of a 25 percent solution.
Table 1. Pure salt concentration and corresponding specific gravity (measured by a hydrometer) at 15°C (59°F).
|
Percent salt |
Specific gravity at 15°C (59°F) |
Percent of saturation |
*Weight of salt kg/m3 (lb/gal) |
|
0 |
1.000 |
0 |
0 (0) |
|
5 |
1.035 |
20 |
51.8 (0.432) |
|
6 |
1.043 |
24 |
62.7 (0.523) |
|
7 |
1.050 |
28 |
73.5 (0.613) |
|
8 |
1.057 |
32 |
84.6 (0.706) |
|
9 |
1.065 |
36 |
95.9 (0.800) |
|
10 |
1.072 |
40 |
107.2 (0.895) |
|
11 |
1.080 |
44 |
118.9 (0.992) |
|
12 |
1.087 |
48 |
119.8 (1.000) |
|
13 |
1.095 |
52 |
131.8 (1.100) |
|
14 |
1.103 |
56 |
154.7 (1.291) |
|
15 |
1.111 |
60 |
166.8 (1.392) |
|
16 |
1.118 |
63 |
178.9 (1.493) |
|
17 |
1.126 |
67 |
191.5 (1.598) |
|
18 |
1.134 |
71 |
204.3 (1.705) |
|
19 |
1.142 |
75 |
217.2 (1.813) |
|
20 |
1.150 |
79 |
230.1 (1.920) |
|
21 |
1.158 |
83 |
243.4 (2.031) |
|
22 |
1.166 |
87 |
256.8 (2.143) |
|
23 |
1.175 |
91 |
270.3 (2.256) |
|
24 |
1.183 |
95 |
284.1 (2.371) |
|
25 |
1.191 |
99 |
293.3 (2.448) |
|
25.2 |
1.200 |
100 |
*Note: Weight of commercial salt required = (weight of pure NaCl from table) ÷ (purity in percent)
CMA will go into solution relatively quickly if vigorously agitated, and especially if warm water is used. Two methods have been used to agitate the solution in a batch system. One method uses paddle mixers in dedicated mixing tanks. For simpler installations, a flat-bottom, 7.5 m3 (2000 gal) polyethylene tank can be fitted with a small electric paddle mixer. This arrangement will provide the necessary agitation. Another method is to keep the CMA particles suspended in the water and in constant motion by the force of moving water. This is accomplished by using a high volume, high pressure pump to recirculate the water until the CMA particles are fully dissolved. This has been successfully used, but the return leg should be submerged in order to minimize the introduction of air. Too much air injection can cause foaming and premature biodegradation.
Washington DOT (WSDOT) has found that the addition of a surfactant (liquid car washing soap) to the solution is effective in suspending insoluble material for weeks. Only about 10 mL (2 teaspoons) are needed per 1000 kg (2205 lb) of solid CMA.
CMA contains about 4 percent by weight of water-insoluble material. These are impurities in the limestone used to make CMA. The particles range in size from 1 to 100 µm (40 to 4000 µin), and most will settle within 24 h after agitation is stopped. The particles are easily suspended by further agitation, and are not prone to caking.
The user needs to determine if the particles will affect performance of pumps and nozzles in the spreader equipment. If so, particles may be removed either by decanting the clear CMA liquid, or by filtering. Decanting can be avoided if a coarse mesh filter that will still prevent clogging of pumps and nozzles is used. Particles removed from the solution are not hazardous. Spreading the residue on sand piles to keep it from freezing has been recommended by some users.
The user needs to evaluate the quantity of liquid CMA needed, and what size batches are appropriate depending on storage capacity, storm frequency, and available personnel resources. This is needed in determining what mixing facilities will be required. An outside CMA mixing facility operated by WSDOT is shown in Figure 13.
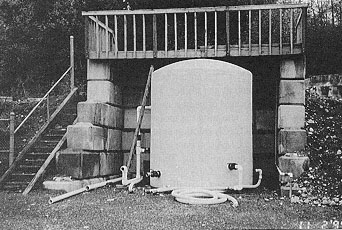
Figure 13. Outside CMA mixing facility operated by Washington DOT.
Chemical solution storage facilities
The decision whether to use inside or outside storage facilities depends on the freezing temperature of the solution and the lowest air temperature expected in the area. If the lowest air temperature is at or below the freezing-point of the solution, then inside storage should be used. However, if that is not possible, heat can be applied either with heat tapes or immersion heaters in the outside storage vessel to maintain the solution temperature above the freezing-point. Some highway agencies have buried the storage vessels and used the earth heat to maintain the temperature above freezing. Buried tanks must meet all code requirements.
The type and kind of storage vessel depends on the solution it contains and whether a secondary containment (e.g., double-walled tank or containment dike) is used. Secondary containment may be required to protect against leakage and pollution: this should be determined from the appropriate regulatory agency. If the solution is corrosive, or if the vessel will ever be used to contain a corrosive solution, the vessel should be made of a noncorrosive material such as stainless steel, glass fiber, or polyethylene.
Depending on the type of chemical solution, agitation or circulation may be required before loading material from the storage vessels to the tank on the spreader. This is especially true of solutions which have additives to reduce corrosion. Agitation can be provided either by paddles within the storage vessel or by circulation using pumps. Generally it is sufficient if this is done approximately 15 min before loading. There are some materials on the market that, if not circulated on a regular basis, will settle to the bottom of the tank. If this occurs, it is very hard to get them back into suspension.
3.1.3 Prewetted solid chemical application capability
The wetting of a solid chemical prior to spreading can improve the effectiveness of the solid chemical in many situations. As described in Appendix B, a solid chemical requires energy to go into solution, and a dry solid chemical particle will remain inert until a liquid film forms. The process of going into solution will be accelerated if a liquid is added to the solid surface. This is only one of the benefits of prewetting. Other advantages include: the solid chemical is spread more uniformly because of less waste from bouncing or traffic action (although not all waste is eliminated); granules adhere to the road surface better; there is a faster and longer-lasting effect; spreading speed can be increased; and in some cases the road surface dries more quickly. The practical result is a reduction in the resources necessary for maintaining the highway since a lower application rate translates into a spreader load covering more area, oftentimes requiring less deadheading (returning to the garage empty) to obtain material.
Solid materials and gradation
Sodium chloride (salt) has been the solid material most commonly used when applying prewetted chemicals in anti-icing operations. The material gradations, however, have ranged from coarse particle size distributions intended for deicing operations to finer sizes more appropriate for anti-icing. In deicing the aim is to get the salt particle to move rapidly through an ice or snow layer to the pavement surface. A large particle will have greater weight and therefore greater success in penetrating this layer. Since it is recommended that an anti-icing treatment be made on pavement with a minimal amount of frozen precipitation, the weight of the particles, and thus the coarse gradation, may not be an advantage. Fine particles have more surface area for an equivalent load weight and will go into solution faster. Their use will decrease the time for a solution to form and cover the entire road surface, and, if the road is clear or well plowed prior to the application, they may be more effective. However, care must be taken to plow ahead of an application when needed, to ensure that the spinner speed is adequate for spreading over the desired road width, and to ensure that the truck speed is not so high that turbulence adversely affects the uniformity of the fine particle spread.
It may be beneficial to have a gradation available at a specific site which is a compromise between those used for deicing and anti-icing in order to reduce the need for multiple stockpiles. The gradation must be selected based on individual site conditions. Several specifications for salt gradation are listed in Appendix A.
Prewetting solutions
The prewetting solutions can be made from sodium chloride (NaCl, salt brine), calcium chloride (CaCl2), magnesium chloride (MgCl2), potassium acetate (KAc), or CMA. Since CaCl2 solutions have a lower freezing-point than salt brine at the recommended concentrations, the possibility of freezing in the storage tank may make its use desirable for very cold sites. Water alone has been used as the prewetting solution, a practical approach for the load-wetting method, but only when used at higher temperatures. Since there may be a risk of freezing in the truck-mounted storage tank or in the supply line to the nozzles or at the nozzles themselves, it is generally better to use a chemical solution. Information on freezing-points of various solution concentrations is given in Appendix B.
CaCl2 has been used as a freeze-proofing additive to salt since the 1940s. Experience has shown the use of CaCl2 to have an advantage over NaCl because it is hygroscopic; i.e., it will absorb moisture from air at a relative humidity (RH) of 42 percent and higher. This serves to keep the salt crystals on the pavement after the bulk of the water has evaporated or been removed by traffic. In contrast, salt by itself will dry up and much will blow away. However, retaining the moisture may also increase tire pick-up. It has been found in field tests that a 20 percent CaCl2 solution applied to salt at the rate of 30 percent by weight of the total mixture is effective. Liquid CMA can be used as a prewetting agent and has the advantage of being essentially non-corrosive and non-polluting. Tests by Minnesota DOT have demonstrated some reduction in corrosion rate of salt when CMA is added. One practice based on several years of experience is to add 30 percent by weight saturated NaCl solution (about 25 percent) to dry salt. Although the chemical used for prewetting will be influential to an extent, it may not be as important as the prewetting/application rate.
Further discussions of solid chemicals, solubility, and chemical solutions pertinent to application of prewetted solids are presented in Appendixes A and B.
3.1.3.2 Prewetting techniques and equipment
Prewetting can be accomplished by either of three methods. First, a prewetting chemical can be injected into material stock pile at a specified dosage. Second, a liquid chemical can be sprayed onto a loaded spreader or on the material as it is being loaded into the spreader. Third, an on-board spray system mounted on the spreader and/or the dump body can add a liquid chemical to the dry chemical at the time of spreading.
Prewetting of stockpile
Wetting of stockpiled salt is performed in the late fall when the temperature of the stockpile drops to approximately 0oC (32oF). The prewetting liquid is usually calcium chloride. One method uses a 42 or 45 percent solution liquid calcium chloride, heated to over 32oC (90oF), hauled to the site by tanker truck and injected vertically into the stockpile at 0.4 to 0.6 m (1.5 to 2 ft) spacing using special spray nozzles which penetrate deep into the pile. The recommended application rate is 30 L (8 gal) of liquid calcium chloride per 1000 kg (ton) of salt. When pumped from the tank truck, the heated liquid calcium chloride flows freely through parts of the stockpile, coating the particles in contact with the liquid within the stockpile with a thin film. The degree of coating and completeness of coverage depends on the injection operator. As the solution is dispensed into the cool stockpile, its temperature rapidly drops. At about 21oC (69oF), crystallization of the solution begins. Deeper into the stockpile, crystal growth continues. As long as the temperature of the stockpile remains well below 21oC (69oF), little runoff of the solution will occur.
The stockpile wetting is performed by the vendor of the liquid calcium chloride. Therefore, the advantages of this method are 1) there is no spray equipment to purchase or maintain, 2) no installation of liquid storage tanks, 3) no training of employees on application procedures. However, there are some known disadvantages to this method of prewetting. Rain or snow on a wetted stockpile will dilute the calcium chloride and cause migration through the pile. Therefore it is essential that stockpiles be covered and placed on impervious asphalt or concrete floors. If the stockpile was built by loader equipment which traveled on top of the material, the injection process may not distribute the solution uniformly through the stockpile. The degree of coating the dry salt during the injection process is highly operator dependent. Frequent working of the pile may be required to keep the pile manageable. Finally, the material cannot be readily carried through a warm season without the solution migrating from the pile. Some highway agencies have abandoned this approach to prewetting because of these disadvantages.
Prewetting of a load or while loading
The second method of prewetting consists of spraying liquid chemical onto a loaded spreader or on the material as it is loaded into the spreader. Application on the load is accomplished by an overhead sprayer with nozzles that dispense the liquid (Figure 14). The driver pulls his truck loaded with dry chemical beneath a timer-controlled overhead spray bar system. A timer button activates a pump which sprays the loaded spreader with a solution. The recommended application rate for a 32 percent CaCl2 concentration is 45 L (12 gal) per 1000 kg (ton) of salt.
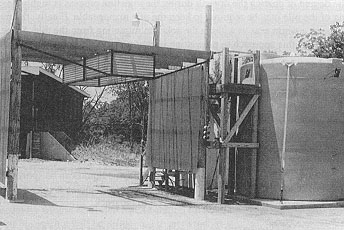
Figure 14. Overhead spraybar for prewetting chemical load in truck.
There are other variations to this second method. For example, the liquid can be sprayed onto each bucketful of salt as it is placed in the truck. Some highway agencies employ a conveyor system, spraying liquid calcium chloride on the salt as it travels up the belt to the truck.
Despite their differences, these variations on the truck-load application method have one important feature in common. First, the equipment is very modest. The basic components for all truck-load systems are a storage tank, a centrifugal pump, piping, an open spray area, a metering device, and the necessary wiring. The cost of a basic truck-load application system with all new components is between $8,000 to $10,000.
The one notable disadvantage of this second method is that it has a very high corrosive effect on the truck equipment. Another drawback is the loaded material has to be completely discharged. The unused portion cannot be left in the truck box or mingled with non-prewetted material. Finally, it is very difficult to get uniform particle coating with this method. Liquid sometimes channels through the load to the truck bed without coating segments of the dry chemical. Other times, too much liquid is used in an attempt to achieve reasonable particle coating.
Prewetting by spreader spray systems
The most common method of prewetting is through the use of on-board spreader spray systems. A spreader equipped for prewetting can apply liquids directly to the material being spread. The prewetting equipment can be an integral part of the spreader design or it can be a system that is added to an existing dry-material spreader. An existing spreader can be modified relatively simply and inexpensively. Both electric and hydraulic spray systems are used. An electric system consists of a 12 V DC electric pump rated at up to 11 L/min (3 gal/min), in-cab controls, one or two nozzles, hoses, spray tank(s) and necessary fittings. The cab controls are generally of two types. The simple type has an on/off switch and a variable speed pump control for increasing or decreasing the liquid material flow rate. Typically, this controller is not ground-speed oriented. The other type of control monitors the amount of granular material being applied and automatically adjusts the liquid flow rate to maintain a constant gallons/ton ratio. The second controller also monitors and displays overall total gallons of liquid pumped and tons of granular material spread per trip and for the entire season. These latter controllers are equipped with their own conveyor/auger sensor. They can generally be used in conjunction with any type of ground-speed control system. Both control systems operate independently of the spread width control.
The hydraulic sprayer is in-line with the conveyor/auger motor which provides a constant relationship with the amount of material being spread. The system includes a liquid spray pump, hydraulic motor, cab controls, nozzle kit, spray tank(s), and necessary hoses and fittings. Generally, a small hydraulic motor is used to drive the product pump and is coupled in series with the conveyor/auger motor. An electrical solenoid valve built into the motor housing is connected to an on/off (system) switch in the cab. An adjustable pressure regulator is located in-line between the pump and nozzles to control the liquid flow rate. An on/off, cab-mounted light displays when the solenoid is engaged. There is also a low pressure sensor to indicate when the liquid level is low or if a problem exists in the system. The system generally includes between two and four brass spray nozzles. Some nozzles have variable orifice openings which provide an extended range of liquid material output.
Generally the on-board spreader tanks are made of molded polyethylene and are provided with a replaceable output-line screen strainer and shut-off valves. Tank capacity is between 0.25 and 0.5 m3 (60 and 125 gal). Some manufacturers can provide stainless steel tanks in lieu of the polyethylene.
The prewetting equipment available from Scandinavian/European sources includes designs that automatically maintain a constant liquid-to-solid ratio regardless of variations in truck speed, spread quantity, or spread width. These prewetting systems are also equipped with hydraulic controls that automatically reduce the solid quantity exiting the conveyor belt 30 percent by weight when the liquid system is engaged. The liquid is pumped through a check valve, then flows by gravity onto the spinner. The dry material and liquid mix as they travel along the curved vanes of the spinner. Nozzles are not used to prewet the material in these designs.
As with all spreaders, periodic calibrations should be made, and any radical deviation in the spreader output compared to the control setting (e.g., running out before the route is completed, or having material remaining) should be investigated. Of some concern is spread uniformity. Although spinner spreaders leave "waves" of material on the road, traffic should distribute a solid or prewetted chemical over the road in both anti-icing and deicing applications. Although a maximum spreading speed of about 64 km/h (40 mph) is achievable, the actual speed used must be selected to ensure uniform material distribution. Also, in order to achieve as uniform a distribution as possible, ground-speed-oriented controls should be used. These modulate the material flow rate as a function of vehicle speed to obtain a constant area coverage.
The prewetting equipment in use by highway agencies is not trouble free. Frequent failures of electric pumps have been reported along with spray nozzle clogging. Also, in some designs, the add-on hydraulic system is not totally compatible with the truck’s hydraulic system. The more sophisticated foreign prewetting equipment also has experienced operational problems. Ruptures have occurred in the liquid chemical feed lines and liquid line couplings have broken or come loose during anti-icing operations.
Chemical solution production and chemical storage facilities
The chemical solution production and storage facilities needed for prewetting operations are generally the same as those needed for liquid applications. However, smaller production/storage facilities may be adequate because of the lesser volumes of the chemical solution that will be required. Storage facilities needed for the solid chemicals are the same as those required for solid chemical applications. If stockpile prewetting techniques are used, storage techniques described above should be used.
3.1.4 Plowing capability
The primary role of snowplowing in an anti-icing operation is to remove as much snow or loose ice as appropriate before applying chemicals in order that excessive dilution is avoided and the applied chemical can be effective. Because the initial chemical treatment should be placed before a significant accumulation, this is generally more important for subsequent operations. However, prior to liquid applications, it is essential that the pavement be cleared of as much snow or loose ice as possible, which may be important even for the initial operation.
If the pavement and snow are cold and dry, and it is apparent that the snow in tire tracks is not adhering to the pavement, then plowing is all that will be necessary.
3.1.4.1 Types of snowplows
There are many types of snowplows. These include one-way front plows, reversible plows, deformable moldboard plows, underbody plows, side wings, and plows specifically designed for slush removal. All plows are hydraulically controlled. In most cases, it takes only a short while to mount and dismount the plows using a quick-change buffer system.
Hydraulically extendible plows have recently been developed. The width of the plow can be extended to the left or right hand side, depending on the manufacturer. These plows are best suited to roads that vary in width. The extendible plows typically allow width adjustment between 3 and 4 m (9 and 12 ft).
Side wing plows can be attached to trucks and motor graders. The one-way front plow and underbody plow can be used simultaneously with the side wing plows.
3.1.4.2 Cutting edges
Cutting edges are available that are made of synthetic polymers, rubber, steel, and carbide inserts. Their performance is dependent on highway and snow and ice conditions. Some highway agencies have experimented to establish the effectiveness of different edges for different conditions. For example, Washington State Department of Transportation has demonstrated that polymer edges are effective for removing slush.
During anti-icing operations the cutting edge should be kept as close to the pavement as possible in an attempt to remove all the snow and slush. Thus, the use of casters or shoes on the plow is not recommended.
3.1.4.3 Slush blades
So-called slush plows have been developed in Sweden and Finland that use two blades, the leading blade having a cutting edge of steel and the trailing blade having an edge of rubber. This design is more effective over a wider range of conditions than is possible with either blade alone. The double blade plows are very good when the consistency of slush varies. Rubber blades are effective only in the removal of wet slush. The wetter the slush, the thicker the rubber blade can be. The slush blades are either spring loaded or hydraulically controlled to maintain pressure on the road surface. These blades cannot remove wet or compacted snow because of their flexibility; they will fold back and become ineffective.
3.2 DECISION-MAKING TOOLBOX
The discussion of the tools in the decision-making toolbox is divided into six major categories: weather forecast information, road and road weather information, nowcasting, traffic information, patrols, and evaluations of treatment effectiveness. Each of these categories is described below.
3.2.1 Weather forecast information
The decision whether or not to initiate a treatment, when to start and what treatment to apply, can only be made if good weather information is available. This includes forecasts for each geographical region of when precipitation is expected to start, what form it will be, the probable air temperatures and the temperature trend during and after the storm, and the wind direction and speed. One source of this information on a regional and national basis is the National Weather Service. Its products must cover the entire gamut of weather users, from agricultural to marine and aviation interests. As a consequence, NWS forecasts are not generally specific enough for maintenance managers to use for decision making for an effective anti-icing program. A good source of locally-specific, timely forecasts is a contract forecast service. Guidelines for selecting a VAMS (Value Added Meteorological Service) along with a sample Request for Proposal for services are included in the report SHRP-H-351 Road Weather Information Systems, Volume 2: Implementation Guide (4).
3.2.2 Road and road weather information
3.2.2.1 Road information
Real-time knowledge of the pavement surface state is necessary for making an informed decision on treatment: the pavement temperature, whether it is wet or dry, and some indication of the concentration of a freezing-point depressant. The most important is pavement temperature. The solubility of all chemicals varies with temperature. The lower the temperature the less the solubility. An ice-control chemical must form a solution in water in order to depress the freezing-point. The pavement temperature will determine if it will form an ice-melting interface at the pavement surface. Air temperature is less important at the critical time of application and immediately following since there is usually a lag between air temperature change and the response of the pavement surface. Nonetheless, the air temperature trend is important to track because pavement temperature will usually follow the air temperature within a few hours depending on the difference in the air temperatures, the amount of solar radiation, wind, and the characteristics of the road.
Pavement sensors accomplish this monitoring and warning function. In addition to their real-time monitoring function, pavement temperature sensors can be used to generate a forecast of pavement temperature trend and warn when it will drop below freezing. This warning can occur several hours before the event, providing sufficient time to plan operations and avoid unnecessary costs.
In addition to measuring temperature most pavement sensors give a relative value of the chemical concentration on the sensor surface based on conductivity measurement. It will serve as a guide to whether some chemical remains on the road and help in making the decision whether or not to retreat. Another capability is available on some of the newest types of pavement sensors: measurement of the freezing-point of the solution on the detector. Its value lies in warning of the refreeze of a chemical treatment which has been diluted by melted snow or ice.
Remote measurement of amount and type of precipitation will guide the maintenance manager in deploying available resources most effectively. It is not unusual for part of a region to be receiving freezing rain, another part snow, and still another no precipitation.
Using the most appropriate chemical and application rate for the condition, scheduling only plowing, or choosing to do nothing can all be informed decisions based on road and weather information (see Section 4 for guidance on operational options).
3.2.2.2 Road Weather Information Systems (RWIS) and their components
Road weather information systems (RWIS) are networks of weather data-gathering and road condition monitoring systems and their associated communications, processing, and display facilities which provide decision information to maintenance managers. The most visible components of RWIS are the roadside installations of system components (Figure 15). A single site, which may have many sensors, is referred to as a remote processing unit (RPU) station. The RPU station typically consists of atmospheric sensors mounted on some form of a tower, sensors embedded in the pavement surface and beneath the surface, and an enclosure which contains data processing capability and communications equipment.
Data from the sensors are formatted at the RPU. They are transmitted to a central processing unit (CPU) where they may be stored, retransmitted to other workstations or locations, or accessed directly. The CPU can be a separate computer or a workstation.
Another component of a RWIS is the data processing and display capability used by the maintenance personnel. The actual system configuration depends on the management structure of the maintenance organization. This component can be a computer workstation in a maintenance facility or at a District or Area headquarters. It can also be a portable computer a manager, supervisor or foreman takes home.
Whoever makes the decisions for allocating resources for snow and ice control should have access to the latest weather and road information. If decisions are made from a central office, perhaps one workstation allocated with the CPU will suffice. If decision making is decentralized, workstations and/or portable computers should be available to the local decision makers for them to access data.
Additional information about RWIS, their selection, procurement, siting, use, maintenance, and calibration can be obtained in the two-volume SHRP report Road Weather Information Systems, Volume 1: Research Report (SHRP-H-350) and Volume 2: Implementation Guide (SHRP-H-351) (5), (4).
3.2.2.3 Role of thermal mapping
Thermal mapping, or thermography, is the process of determining thermal profiles of road surfaces using infrared sensors. The measurements are typically made in the early morning hours, when there is the least change in the pavement temperature during the measurement process. They are also made under different atmospheric conditions, since the radiation balance at the surface is related to the atmospheric conditions, including cloud cover, wind speed, and precipitation.
A variation of thermal mapping is called road climatology. Additional data are acquired when measuring pavement temperature, including air temperature, relative humidity, and climatological characteristics of the pavement environment. The additional data are input to a short-range (up to 4 h) forecasting model for pavement temperature.
Thermal mapping of highway segments has been conducted in several States, including Washington, Nevada, and Minnesota. The data from thermal mapping have assisted in siting RPU stations, forecasting pavement temperatures for locations where no RWIS sensors exist, and for developing snow- and ice-control strategies. Other potential locations for thermal mapping include those areas where anti-icing operations are used, where reduced chemical areas exist, or where a significant number of different microclimates exist in a given area. Thermal mapping may also point to representative RPU locations that can eliminate the need for one or more sites.
Thermal mapping profiles can be used to infer pavement temperatures between sensor locations where the temperatures are known. An extension of this process is to forecast temperatures along the roadway based on the forecasts of temperatures at known points. This approach has been used in the United Kingdom in areas where frost or ice formation on the roads is a concern.
Better routing or allocation of maintenance resources and personnel is possible based on thermal mapping. The data can allow staging of responses to only those road segments expected to be below freezing. It can also indicate certain areas or locations that may not need attention.
Research has indicated that thermal information from the road environment can be obtained using relatively inexpensive hand-held radiometers (2, 5). Vehicle-mounted instruments for measuring pavement temperatures are already used by some State highway agencies.
There is some thought that thermal mapping should be considered when variations of pavement temperature greater than 5oC (9oF) are possible, or when the road elevation changes more than about 200 m (650 ft) over the segment length of interest. These "rules of thumb" are for general guidance and have not been validated by research data.
3.2.3 Nowcasting
Nowcasting refers to the use of real-time data for short-term forecasting. It relies on the rapid transmittal of data from RWIS installations, radar, patrols (see Section 3.2.5), and any other information source for making a judgment of the probable weather and pavement condition/temperature over the next hour or two. Nowcasting is one important tool for making the decision of when to call in personnel. Mobilization timing may vary among sites, therefore the frequency of weather information updating required for a nowcast will also vary with the site. Nowcasts can be provided by a weather service or performed by the maintenance manager. Specially trained maintenance managers in some highway agencies already perform this duty using the necessary information available from a variety of sources.
3.2.4 Traffic information
Vehicles can affect the pavement surface in several ways: tires compact snow, abrade it, displace or disperse it; heat from tire friction, engine, and the exhaust system can add measurable heat to the pavement surface. As described previously, they can also result in applied chemical being blown from the pavement. As a consequence, they can influence, both positively and negatively, the effectiveness of anti-icing treatments, and should be considered in the decision-making process. The traffic information most important for making operational decisions is the variation of traffic rate throughout a 24 h period.
3.2.5 Patrols
There is no substitute for visual observation of weather conditions and conditions of the pavement surface. Observations remain an important tool for making operational decisions even when an agency has access to and experience with new technology such as RWIS. Use of patrols for this purpose can be highly effective. Though the State or local highway patrol can fulfill this role, trained maintenance personnel are better prepared to judge the severity of conditions and to make or recommend corrective action.
3.2.6 Evaluations of treatment effectiveness
Maintenance decisions should not be based on a rigid, automatic basis but rather on the assessment of a need. In contrast to prescribing that chemicals be applied, or plow runs be made every hour or two or other fixed interval, decision on treatment need can be based on a number of information sources. The first and most obvious is the visual observations of precipitation/weather and pavement conditions from patrols, as discussed above, and from operators. The second is an indication or the measurement of chemical concentration on the pavement. The third is the measurement of frictional resistance to sliding.
The availability of chemical concentration indicators appears to enhance the timing of subsequent applications by providing indications of the dilution of the chemical. A manager can time the reapplication of chemicals so that the operation is complete before the freezing-point of the brine on the pavement surface starts to climb and, especially, before it reaches 0oC (32oF). Where decision makers have confidence in these data, they can be used as a basis for establishing cycle times of the repeat applications for different conditions.
Measurement of friction was used successfully in the SHRP and FHWA anti-icing projects. An agency may find it reasonable to establish this as a technique used during patrols. There are many devices for measuring friction. Skid trailers are commonly used for the measurement of the coefficient of friction, but for various reasons related to safety and equipment deterioration, they are not normally used on snow-covered pavements. Specialized vehicles incorporating a fifth wheel, which measures the increase in force when braked at a controlled slip rate, are available, but high cost has limited their use mainly to airports. A low-cost device was used in both the SHRP and FHWA test programs because it can be installed in most any vehicle and can produce reliable measurements. It gives a direct readout of friction coefficient when the vehicle is hard-braked from 65 km/h (40 mph). Its repeatability is acceptable for treatment analysis and decision support purposes, provided the device is calibrated and operated in accordance with the manufacturer’s specifications. Because it requires hard braking, however, it is not suitable for use in heavy traffic.
In addition to evaluations during a storm, it is beneficial for the personnel of each maintenance area to conduct a post-storm evaluation of the treatment effectiveness. This can help identify areas needing improvement and changes that can be made in the treatment strategy. A post-season review of treatment effectiveness is likewise helpful. It can help identify where changes are needed in equipment, material, and route configurations, and can begin a process of engineering an anti-icing program to fit the exact needs of a site or agency. It can also help identify where changes in personnel procedures and training are needed to improve the effectiveness of the winter maintenance program.
3.3 PERSONNEL TOOLBOX
The discussion of the tools in the personnel toolbox is divided into two major categories: trained personnel for anti-icing decision making and operations, and deployment of personnel. Each of these is described below.
3.3.1 Trained personnel for anti-icing decision making and operations
It is essential for effective implementation of an anti-icing program that personnel be trained in the details of the workings of the program. Anti-icing techniques and operations may be so foreign to many operators and managers that old ideas must be banished before a workable program can be started. Everybody resists change, but change in most cases is what is required for an anti-icing program to be successful. An anti-icing program will necessitate more information for making an informed decision and may involve different methods and materials than do conventional methods. This will require an emphasis on training. This training can be accomplished by a consultant or highway agency staff using this manual and other material developed under FHWA and SHRP studies (1,2). Workshop material being produced under a current FHWA study (6) can be used also during the training.
3.3.2 Deployment of personnel
Deployment of personnel for anti-icing operations involves improved standby and call-out procedures. Use of modern weather forecasting information will provide more time in advance of a storm to plan operations. With better information, personnel can be advised of standby status with more certainty of need. Personnel on standby must abide by agency requirements regarding alcohol consumption, availability, and rest. Since many agencies may pay personnel for standby time, reducing unproductive call-outs and standby time will represent large savings.
Once the decision has been made that a snow and ice control operation is necessary based on accurate and timely weather information, crews must be called out. The necessary weather information needed to make the decision to initiate call-out procedures must be provided in a timely manner to ensure adequate lead time for mobilization of resources. Lead time may vary by site, thus weather information needs may also vary by site. This is especially important for anti-icing operations as the timing of the initial treatment is critical. By minimizing the mobilization time, total crew time may be reduced or crew availability may be extended. A well-organized call-out system must be in place to mobilize crews within the required time. Automated telephone calling systems can assist in streamlining the call-out process.
This section presents general recommendations for successful anti-icing practices that can be employed for various combinations of precipitation, pavement temperature, traffic volumes, and mandated levels of service. It presents information that will assist in the development of a systematic anti-icing program, and complements Section 3 on the capabilities, information sources, and procedures that make up the tools of an anti-icing program. It contains four major components: initial operations, subsequent operations, special considerations, and post-storm assessment of operations.
The section is intended as a companion and background document to Appendix C, which presents specific and concise recommendations for anti-icing operations for six weather events. These are:
Light Snow Storm
Light Snow Storm with Period(s) of Moderate or Heavy Snow
Moderate or Heavy Snow Storm
Frost or Black Ice
Freezing Rain Storm
Sleet Storm
Guidance on maintenance actions for each event is provided in the Appendix C tables for several pavement temperature ranges and for initial and subsequent operations. Temperature trend, an important factor, is also indicated. Solid, liquid, and prewetted solid chemical application rates are suggested where appropriate. These rates are not to be considered as fixed values but rather the middle of a range to be selected by an agency according to its local conditions and experience. Traffic volumes have not been found to have a consistent or dominant influence on pavement condition or traction to suggest varying chemical application rates except in the case of frost and black ice, and that category is the only one incorporating traffic as an operational consideration. Special considerations associated with maintenance actions are listed in the Comments and Notes given on the tables.
The guidance presented in this section and in Appendix C is based upon the results of 4 years of anti-icing field testing conducted by 15 State highway agencies and supported by the Strategic Highway Research Program (SHRP) and the Federal Highway Administration (FHWA). It has been augmented with practices developed outside the U.S., where necessary, for completeness. However, no short discussion or list of recommendations can completely cover the range of conditions facing agencies continent wide. Therefore every agency is encouraged to use the guidance of this document as a starting point for developing its own anti-icing program, and to modify the recommendations when necessary in order to accommodate local experience, specific site concerns, and agency objectives. Careful recording of conditions and the operations made in response will provide the basis for fine-tuning the program for specific service levels and conditions.
4.1 INITIAL OPERATION
The initial anti-icing operation is most often the application of a chemical freezing-point depressant to the pavement before enough snow has accumulated to keep the chemical from reaching the pavement. However, before this action is taken, information about the nature and characteristics of the anticipated storm should be assembled and a decision made concerning the action. Each of these steps is discussed next.
4.1.1 Information assembly
Several pieces of information need to be assembled upon first notice that a winter storm or frost/black ice event may affect the maintenance area. This information includes weather forecasts, weather radar data, satellite data, local road condition and RWIS data, pavement temperature forecasts, and any RWIS data from areas outside the immediate maintenance jurisdiction that might have already have been affected by the approaching storm. The information must be reviewed to estimate when and where the event will begin, its extent, and severity.
4.1.2 Decision point
The decision on whether or not to initiate a treatment, when to start it and what type of treatment to apply can be made after the review is made of the information assembled. The decision is based on when precipitation is expected to start, what form it will be, the probable air and pavement temperatures, the anticipated trend of the temperatures, the expected sky conditions, the wind speed and direction, and the intended timing of the treatment.
4.1.3.1 Apply chemical
Either dry solid chemicals, liquid chemicals, or prewetted solid chemicals can be used as an initial anti-icing treatment. Whichever is used, the timing of the application should be consistent with the underlying objective of preventing the formation or development of bonded snow or ice, and should reflect an underlying readiness consistent with a preventive strategy. That is, it should be made in anticipation of or in prompt response to worsening pavement conditions. Applications in advance of snowfall are not necessary for preventing bonded snowpack, but early applications when the pavement condition is no worse than wet, slushy, or lightly snow covered are for the most part necessary for anti-icing success. As this may not always be possible, for example because of a limited fleet or heavy traffic, pretreating the road before a snowstorm may be the only way to ensure that all areas are treated before conditions deteriorate.
Residual chemical from previous operations has a short-lived effect on highway conditions at the beginning of storms, and should not be relied upon for timing of initial anti-icing operations without independent indications of adequate chemical concentration.
Recommendations for use of liquid chemicals
Guidance for initial liquid treatments is given in Appendix C for four events: light snow storm; light snow storm with period(s) of moderate or heavy snow; moderate or heavy snow storm; and frost or black ice. The use of a liquid is not recommended during either a freezing rain or sleet storm because of the large quantity needed to retain an effective concentration. The suggested application rates are the equivalent dry chemical rates. All are given only for pavement temperatures at -5oC (23oF) and above because of the ineffectiveness of the indicated rates at pavement temperatures below -5oC (23oF).
For snowstorms, initial liquid applications can be made either as a "pretreatment" in advance of the storm or as an "early-storm treatment," i.e., soon after snowfall has begun and/or when the pavement temperature is dropping toward freezing. A pretreatment can be made well ahead of a storm as long as the storm does not start out with above freezing temperatures and rain, washing the chemical away. In the case of early-storm treatment, the application may be made onto dry, wet, light slush, or lightly snow covered pavement. Late applications onto pavements with more than a light covering of slush or snow can result in excessive dilution of the chemical, and risk failure. These should always be coordinated with plowing.
Relative to conventional practices in which surfaces are not treated until later in a storm, benefits from liquid pretreatments can include higher friction and better pavement conditions early in a storm. These benefits are generally short-lived, however, and should not be expected over a long period. Subsequent chemical applications should be made as soon as conditions begin to deteriorate. In essence, pretreatments can be thought of as "buying time" in the earliest stages of a storm until subsequent chemical applications become effective.
For preventing the formation of frost or black ice -- caused by radiational cooling of the pavement in the presence of high humidity -- the chemical should be applied in advance of the expected time of ice formation so that the water component of the brine will evaporate or be removed by traffic action. This will leave only the chemical on the road surface, and thus result in the greatest concentration when frost or black ice conditions occur. Of the recommended frost or black ice treatments given in Appendix C, traffic condition is a dominant factor only in the temperature range of -2 to 2oC (28 to 35oF).
As an example of frost prevention, one agency has found that a solution of 27 percent MgCl2 can be applied to bridge decks in valley areas to prevent preferential icing (frosting) conditions. The liquid is applied at the rate of 100 L (25 gal) per lane-mile at speeds up to 50 km/h (30 mph). Depending on traffic and weather conditions, the residual chemical can prevent frosting conditions for about one week on low-volume roads and 3 to 4 days on higher volume freeways. Appendix C includes this experience as guidance, presenting the application rate in terms of the weight of the dry chemical in the applied solution.
Recommendations for use of solid and prewetted solid chemicals
Guidance for initial prewetted solid chemical applications is given in Appendix C for all of the events. Guidance for initial dry solid chemical treatments is given for three events only: light snow storm; light snow storm with period(s) of moderate or heavy snow; and moderate or heavy snow storm (dry solid chemical is referred to as solid chemical in the Appendix C tables). The differences in the recommendations for solid and prewetted solids are primarily a result of the additional benefits that prewetting provides and the limitations on the use of solid chemicals when insufficient moisture or accumulation is present on the road. These have been discussed in Sections 3.1.1 and 3.1.3. For some conditions identified in the Appendix C tables, prewetting is not listed as a recommended maintenance action even though dry solid chemicals are. These are cases where sufficient moisture is present to trigger the effectiveness of the dry chemical. Under such conditions, however, there would be no loss of effectiveness in applying prewetted solids.
For precipitation events, prewetted solid applications, like liquid treatments, can be made either in advance of the storm or as an early-storm treatment. In the latter case, the application can be made onto dry, wet, slush, or lightly snow covered pavement. It should be completed before accumulation or pack bonds to the pavement. Applications onto dry pavement, either as a pretreatment or early-storm treatment, should be monitored to avoid excessive loss of material. Late applications onto pavements with more than a light covering of slush or snow can result in excessive dilution of the chemical, and should be coordinated with plowing. Where there is sufficient moisture after snowfall has begun, dry solid chemicals can be applied.
Application of dry solid chemical onto dry pavement is not recommended, and therefore should not be used as a pretreatment. Timing of an initial dry solid chemical application for snowstorm events is therefore critical; it should be made as soon as possible after sufficient precipitation has fallen to prevent loss, but before snowpack or ice bonds to the pavement.
4.1.3.2 Plow
If the pavement and snow are cold and dry, and it is apparent that snow in tire tracks is not adhering to the pavement, plowing is all that will be necessary to remove accumulation. If residual chemical or pavement temperature is high enough to form some liquid, wetting the snow or causing slush, then plowing with an appropriate cutting edge or slush blade is recommended (see Section 3.1.4).
4.1.3.3 Do nothing
When the pavement is cold (below -9.5oC (15oF)) and new or blowing snow are light and cold, traffic and wind (speeds of 25 km/h (15 mph) or higher) may be sufficient to prevent accumulation and compaction in tire tracks. In this case, application of any chemical, even that added as freeze-proofing to an abrasive, may create rather than cure a problem. Once a wet surface develops where before it was cold and dry, the dry snow can adhere and begin to build up. Prompt removal with a plow may prevent pack from developing, but this situation may have been avoided by refraining from chemical application. If the weather forecast is for rising temperatures, however, chemical should be applied before snow becomes wet with the potential of forming pack. Application should be made when the temperature rises high enough for the chemical to act rapidly, usually above about -5oC (23oF). Application can be made at temperatures as low as -9.5oC (15oF) if a rapid rise in temperature is forecast.
4.2 SUBSEQUENT OPERATIONS
An initial application of chemical may suffice for some conditions and short duration events, but it is far more likely that further treatments will be required during a storm. In many cases these do not differ from initial treatments, although other considerations become very important such as the coordination of the application with plowing.
4.2.1 Monitoring of conditions
It is important that roadway and weather conditions, weather forecast updates, and RWIS data be closely monitored once the initial anti-icing operation has taken place. Special attention should be paid to pavement temperature and trend and to changes in precipitation type and intensity. This information, plus observations of precipitation, observations of pavement conditions, and evaluations of treatment effectiveness (as discussed in Section 3.2.6) are needed to determine when, or if, additional anti-icing treatments are necessary. Only by the systematic use of available information can the most efficient anti-icing actions be taken over the course of a storm.
4.2.2.1 Apply chemical
As for the initial operation, guidance for solid, liquid, and prewetted solid chemical applications is given in Appendix C for subsequent operations that may be necessary. Again the guidance for liquid applications is limited to four events: light snow storm; light snow storm with period(s) of moderate or heavy snow; moderate or heavy snow storm; and frost or black ice. Also, guidance for prewetted solid chemical applications is given for all of the events, whereas guidance for dry solid chemical treatments is given for three events only: light snow storm; light snow storm with period(s) of moderate or heavy snow; and moderate or heavy snow storm. Much of the discussion above for the initial chemical application is pertinent to the subsequent application.
Whichever treatment is used, the timing of subsequent applications, like the initial application, should be consistent with the underlying objective of preventing the formation or development of bonded snow or ice, and should reflect an operational readiness consistent with a preventive strategy. That is, they should be made in anticipation of or in prompt response to worsening pavement conditions.
For snowfalls, an initial anti-icing treatment may be all that is necessary to cope with a light or short duration event. There will be little dilution of the chemical, so the freezing-point may not reach the pavement temperature. When snowfall continues, and pavement temperature is about -9.5oC (15oF) or higher, subsequent treatments may be required to prevent the formation or development of pack or bonded pack. In such a case, the snow should be plowed off as completely as possible before chemical is reapplied, allowing the necessary small amount of chemical to reach the pavement surface.
In snow storms with generally steady precipitation and pavement temperature conditions, subsequent chemical operations made at regular intervals are generally adequate. However, in storms with significant changes in precipitation and pavement temperature conditions, operations will likely be at irregular intervals. In either case, systematic operations using all available decision-making tools should be conducted. Subsequent chemical applications that are made in prompt response to changing conditions can improve friction and pavement conditions. Subsequent chemical applications that are made in anticipation of changing conditions can prevent deteriorating conditions or mitigate their effects.
A limited period of heavier snow in an otherwise light snow storm should be treated as "a storm within a storm." That is, chemical operations should be conducted just prior to or at the beginning of the intense snow period to reduce the likelihood that snowpack will develop or be sustained by a strong bond, and to increase the likelihood that plowing operations can readily remove any packed snow that may develop. Use of reliable short-term forecasting tools would facilitate the timing of these operations, but they would otherwise be routine extensions of the responses to heavy snow seen in current snow and ice control practice. The result may not only be the prevention of a strong bond between the packed snow and the pavement, but also the prevention of excessive reductions in friction that precede the development of snowpack. Guidance for the weather event "Light Snow Storm with Period(s) of Moderate or Heavy Snow" was developed specifically to underscore the importance of being ready for these heavy snow periods and the poor road conditions that often result.
Although subsequent liquid applications can be successful, operational constraints such as the capacities of truck tanks and storage facilities, as well as operational preferences, may lead to anti-icing chemical operations that include transitions from liquid to solid applications. In such cases, the transition should be anticipated to allow continuous anti-icing operations and to avoid operational delays, and the solid chemical operations should be performed according to anti-icing practices.
4.2.2.2 Plow
Plowing passes should be made as necessary to prevent build-up of a compacted snow layer. Before applying any more chemical, the pavement surface should be cleared of frozen precipitation as best as possible to allow the small amount of chemical to reach the pavement surface. This is very important for the application of liquids.
As described for the initial operation, if the pavement and snow are cold and dry, and it is apparent that snow in tire tracks is not adhering to the pavement, plowing is all that will be necessary to remove accumulation.
4.2.2.3 Do nothing
If the initial or previous anti-icing treatments have done their job, the pavement temperature is around
-2oC (28oF) and holding steady or rising, and there is no additional precipitation coming down or forecast, there may be no need for further action. This is especially the case when the pavement temperature is above 0oC (32oF) and steady or rising, whether it is during or after precipitation. Recognition of such conditions, and communication of these conditions to operators, can result in significant material savings. However, it is important to monitor conditions closely when pavement temperature is below or slightly above 0oC (32oF), and to be aware of the potential for surprise freeze-ups.
4.3 SPECIAL CONSIDERATIONS
Several special factors influence choice or timing of an operational treatment. These are introduced or amplified here.
4.3.1 Traffic condition
Other than the influence that traffic volume has on the assignment of level of service, the influence of traffic on anti-icing storm operations comes primarily from the variation of traffic rate throughout the period of a storm or icing event. Although traffic density has an effect on friction, this is less important and not as direct or consistent as the effects of precipitation type and rate and pavement temperature. In fact, anti-icing snowstorm operations have been shown to be successful in high volume rush hour traffic as well as low volume middle-of-night traffic. Because of this, and because traffic can have both positive and detrimental effects on road condition during anti-icing operations, traffic rate has not been included as a variable in the anti-icing guidance provided in Appendix C, except in the table for frost or black ice. The routine use of traffic information should be mostly to ensure that storm operations are completed ahead of rush periods in order to avoid delays which can lead to bonded pack or ice. This is reflected in the Notes provided in the Appendix C tables. Local experience or level of service may warrant further incorporation of traffic information within the anti-icing operational guidance. If so, the Appendix C tables can be appropriately modified.
4.3.2 Wind
Experience has shown that crosswind speeds in excess of about 24 km/h (15 mph) may cause local drifting across a pavement and retention of snow if, for example, the pavement is wet. The threshold wind speed at which this becomes a problem will vary widely with road siting and other conditions. Maintenance personnel should be alert to the conditions that may cause this interception of snow and incorporate the information in operating procedures. For liquid applications, when a decision is made to apply chemical during windy conditions, experience has shown that adjusting the sprayer closer to the pavement can be successful in avoiding loss and achieving the desired application.
4.3.3 Hybrid precipitation events
Few storms will fit neatly into specific classifications based on intensity, duration, and type of precipitation. The operational treatment used early in a storm may have to be modified during the course of the event or operation. This is particularly critical when a light snowfall is interspersed with one or more periods of heavy snowfall. "Light Snow Storm with Period(s) of Moderate or Heavy Snow" has been defined for this case in Appendix C. However, other "hybrid" events are not specified in the remaining Appendix C tables, which are organized according to the type of storm or event. In the occurrence of changing precipitation conditions, maintenance managers should be prepared to base operational decisions on more than one of the tables.
4.3.4 Prewetting quantities
In theory, only sufficient liquid to wet every particle of dry solid chemical is required for prewetting. The actual rate to achieve this wetting will vary with the particle size distribution. In practice it has been found, for example, that 37 L (10 gal) of 23 percent concentration sodium chloride solution will be sufficient for a 1000 kg (1 ton) of dry chemical of coarse gradation. Some agencies have used three times this quantity so that the material is applied as a slurry in order to reduce losses by traffic action.
4.3.5 Development of snowpack and bond
Even when anti-icing operations are performed successfully, a snowpack may still develop and a bond may form between the pack and pavement. Technically this generally implies that deicing is necessary. However it is often observed in practice that the previous anti-icing treatments have inhibited the development of a bond, resulting in a weak bond that is easily broken, leading to a rapid return to acceptable pavement conditions. Those charged with developing anti-icing programs should recognize that bonded snowpack can occur even when anti-icing is "successful," but that it will not usually have the strength of bonded pack observed during traditional deicing operations. Indeed, the stated objective of anti-icing is not only to prevent formation of a bond, but to prevent development if one does form.
If during anti-icing operations rapidly changing climatic conditions or delays in operations lead to strongly bonded snow or ice, maintenance managers should be prepared (1) to utilize deicing techniques to break the bond to return the road to acceptable conditions, and (2) to apply abrasives if a rapid increase in friction coefficient is required in the interim. However, once acceptable road conditions are recovered, a return to preventive anti-icing operations would be appropriate.
4.3.6 Use of abrasives
Abrasives may be necessary when a rapid increase in friction coefficient is required, particularly at pavement temperatures so low that chemical action is slow, and, as discussed above, in conditions where snow or ice is strongly bonded to the pavement and cannot be removed rapidly. Several tables in Appendix C contain low pavement temperature conditions that may warrant abrasives use, most directly in the tables for frost/black ice events and freezing rain storms. However, abrasives are not ice-control chemicals, and will not support the fundamental objective of anti-icing when applied either straight or in a mix with chemicals. Because of this, and because of evidence that they are generally ineffective when used routinely in anti-icing operations, they should not be a customary operation of an anti-icing program outside of the low pavement temperature conditions presented in Appendix C. However, even in these conditions, chemical typically added as freeze-proofing to abrasives may create a problem by causing a wet pavement surface. As described previously in Section 4.1.3.3, once a wet surface develops where before it was cold and dry, the dry snow can adhere and begin to build up. Maintenance crews should be aware of this possibility.
4.4 POST-STORM ASSESSMENT OF OPERATIONS
Lessons can be learned from both the successes and failures of any winter maintenance operation. Anti-icing operations are no exception. Improvements in operations, and even in equipment, can be identified and implemented through a post-storm assessment of the practices and treatments used. It is important that all levels of maintenance personnel, from district level supervisors to equipment operators, be involved in these evaluations. Many times, what is witnessed during a storm by a supervisor is not readily seen by an operator, and vice versa. As part of the post-storm assessment, it is suggested that the highway agency track the cost and effectiveness of the anti-icing program, and, if possible, do the same for conventional snow and ice control operations in comparable areas. This process should include evaluations of treatment effectiveness, as discussed in Section 3.2.6, and examinations of costs. Techniques for these evaluations can be based upon an agency’s current storm data collection practice, or upon those used for FHWA T&E 28 (1).
The cost data of a storm or weather event can be organized according to the total costs for materials (chemicals and abrasives), labor, and equipment employed in operations. For chemicals, this includes the purchase price, transportation to storage site, storage, truck loading, handling and mixing of solid chemicals, and solution preparation. For abrasives it should include both material costs and any clean-up costs. Labor costs should be fully burdened and include overtime, while equipment costs would be the agency’s hourly rates times the hours used. Other costs can be considered, if appropriate. These include the cost of dispatchers, costs of specialized equipment, cost of patrols, etc.
The costs and effectiveness measures of the anti-icing and conventional operations need to be recorded separately for each highway section or route considered. The cost per lane mile can then be calculated for each type of operation, effectiveness measures can be evaluated, and the relative success of the two operations compared.
While the implementation of systematic anti-icing practices can be thought of as a means for maintaining roads in the best conditions possible during a winter storm, and a way to do so in an efficient manner, it should not be thought of as an improvement that will automatically result in reduced overall costs. Most importantly, anti-icing techniques provide the potential for maintaining roads in the best conditions possible during winter storms. Cost savings will depend on the current practice: for example, what level of service governs, what materials it uses, whether it is more deicing than anti-icing, and what information sources it uses. Examples of success include providing the same level of maintenance effectiveness at less total cost, and providing a higher level of maintenance effectiveness at the same cost. As these examples illustrate, for those examining the costs or success of anti-icing operations, an examination of both costs and level of maintenance effectiveness is important.
Information is presented in this appendix on the properties of five chemicals used for anti-icing treatments and instructions for preparing various liquid concentrations. The five chemicals are calcium chloride, sodium chloride, magnesium chloride, calcium magnesium acetate, and potassium acetate. They are listed here with their eutectic temperatures and concentrations:
|
Chemical |
Eutectic temperature
|
Eutectic concentration
|
|
calcium chloride (CaCl2) |
-51 (-60) |
29.8 |
|
sodium chloride (NaCl) |
-21 (-5.8) |
23.3 |
|
magnesium chloride (MgCl2) |
-33 (-28) |
21.6 |
|
calcium magnesium acetate (CMA) |
-27.5 (-17.5) |
32.5 |
|
potassium acetate (KAc) |
-60 (-76) |
49 |
No information is provided on the corrosive properties or environmental aspects of the chemicals. Discussions of these items can be found in the literature. Likewise no cost data for the chemicals are given. These data can be obtained readily from distributors.
Two methods are used to manufacture commercial CaCl2: extraction from natural brines obtained from deep wells, principally in Michigan; and by a chemical process, the Solvay process, in which sodium chloride is reacted with calcium carbonate to produce sodium carbonate (soda ash) and calcium chloride.
The American Society for Testing and Materials (ASTM) has prepared two standards for calcium chloride: D 98 Specification for Calcium Chloride, and E 449 Standard Test Method of Analysis of Calcium Chloride.
A.1.2 Preparation of liquid CaCl2
Solid calcium chloride dissolves readily in water with little agitation required. Considerable heat is given off when it dissolves. Two methods of mixing can be used to obtain a specific concentration of liquid CaCl2. Method 1 is used if the volume of the mixing container is known. Use Method 2 if the volume of the mixing container is not known. Each of these methods is described below. For both, the water temperature should be below 20oC (68oF).
A.1.2.1 Method 1 (Known mixing container volume)
From the "per volume solution" column of Table 2 determine the weight in kg (or lb) of solid calcium chloride required to make 1 m3 (or 1 gal) of solution at the desired concentration level. This value, multiplied by the volume of the container in m3 (or gal), gives the total weight of calcium chloride required.
Fill the container approximately 2/3 full of water, then add the required calcium chloride gradually while stirring carefully with a paddle by hand, with a mechanical agitator, or with an air bubbler.
After the calcium chloride has completely dissolved, add water to the container to bring the level to the working volume and then agitate the solution slowly until a uniform mixture is obtained.
|
%CaCl2 actual |
Weight CaCl2 77% flake |
Crystallization |
Weight per unit | |
|
per volume solution kg/m3 (lb/gal) |
per volume water kg/m3 (lb/gal) |
temperature
|
volume of solution
| |
|
10 |
139 (1.16) |
146 (1.22) |
-5.4 (22.3) |
1085 (9.06) |
|
15 |
218 (1.82) |
238 (1.99) |
-10.3 (13.5) |
1133 (9.46) |
|
20 |
303 (2.53) |
344 (2.87) |
-18.0 (-0.4) |
1185 (9.89) |
|
25 |
397 (3.31) |
471 (3.93) |
-29.4 (-21) |
1234 (10.3) |
|
29.8* |
491 (4.1) |
621 (5.18) |
-55.0 (-67) |
1288 (10.75) |
|
30 |
498 (4.16) |
627 (5.23) |
-46.0 (-50.8) |
1294 (10.8) |
*Note: this is the "eutectic" point, i.e., the concentration that results in the lowest temperature (-55oC (-67°F)) at which a solution can exist while remaining completely liquid.
A.1.2.2 Method 2 (Unknown mixing container volume)
Put a measured volume of water in the container to no more than 2/3 of the container capacity.
Dissolve in it the required weight of calcium chloride given in the "per volume water" column of Table 2 for each cubic meter (or gallon) of water used. Add the calcium chloride slowly to the water with agitation.
When completely dissolved, the solution will have the desired concentration.
For those mathematically inclined, the following formula can be used to determine the volume of water required for a given level of concentration.
| m3 of water required to make a solution of a desired concentration |
= |
Example: to make a 20 percent solution from 500 kg of flake CaCl2 (this is typically 78 percent concentration),
| 500 x 78 20 |
- 500 | ÷ 1000 = 1.45 m3 water |
(In English units:
| gal of water required to make a solution of a desired concentration |
= |
Example: to make a 20 percent solution from 1000 lb of flake CaCl2 (this is typically 78 percent concentration),
| 100 x 78 20 |
- 1000 | ÷ 8.34 = 348 gal water |
A word of caution. Calcium chloride when dissolved gives off heat. This heat of solution causes the brine to expand and occupy more space than it will after it cools. That’s why the container in Method 2 is filled to no more than 2/3 capacity. For example, additional tank capacity of approximately 90 L (23 gal) for every
4 m3 (1000 gal) of 20 percent solution is required. This will increase slightly to 100 L (26 gal) for every
4 m3 (1000 gal) of a 34 percent solution.
Always add the calcium chloride to the water. If you put the calcium chloride in the container first and then add water, the chemical may form a solid mass which is difficult to dissolve completely.
Sodium chloride has been used as an ice-control chemical on roads since early in this century. It is produced by three processes: rock salt is mined by conventional hard rock mining equipment and techniques; solar salt is produced by the evaporation of sea water and may contain only a small amount of impurities; and evaporated or solution or vacuum salt, a very pure form made by drying under vacuum the solution resulting from injection of water into deep underground deposits. Most salt used for highway applications in the United States is rock salt, though some solar salt is produced in several western states and some is imported into the eastern US. Naturally occurring rock salt is the mineral halite, and usually contains between 1 percent and 4 percent impurities, mostly gypsum, shale, dolomite, and quartz.
The ASTM designation for salt used for highway ice control is D 632 Standard Specification for Sodium Chloride.
Two gradations of salt are designated by the ASTM standard as shown in Table 3. Similar but slightly different gradations are used by the British. These along with the Swedish and Finnish gradations are shown below, in Tables 4, 5 and 6, respectively.
Table 3. Gradation of salt specified by ASTM D 632.
|
Sieve size |
Weight % passing | |
|
Grade 1 |
Grade 2 | |
|
19.0 mm (3/4 in) 12.5 mm (1/2 in) 9.5 mm (3/8 in) 4.75 mm (No. 4) 2.36 mm (No. 8) 600 µm (No. 30) |
. . . 100 95 to 100 20 to 90 10 to 60 0 to 15 |
100 . . . . . . 20 to 100 10 to 60 0 to 15 |
.
|
Type and grade of salt |
BS 410 test sieve |
Percentage passing test sieve | |
|
Rock salt |
Coarse |
10 mm 6.3 mm 2.36 mm 300 mm |
100 75 to 95 30 to 70 0 to 20 |
|
Fine |
6.3 mm 2.36 mm 300 mm |
100 30 to 80 0 to 20 | |
|
Vacuum salt and marine salt |
Coarse |
10 mm 1.18 mm 150 mm |
100 0 to 80 0 to 10 |
|
Fine |
1.18 mm 150 mm |
100 0 to 30 | |
Table 5. Swedish gradation for salt.
|
Sieve size,mm |
Weight % passing |
|
3 2 1 0.5 0.16 |
95-100 65-100 26-50 5-26 0-5 |
Table 6. Finnish gradation for salt.
|
Sieve size, mm |
Weight % passing |
|
5 4 3 2 1 0.5 |
100 90-100 70-100 40-90 15-55 3-25 |
A.2.2 Preparation of liquid NaCl
Two methods of mixing can be used to obtain a specific concentration of liquid NaCl. Method 1 is used if the volume of the mixing container is known and a desired volume of salt brine is to be produced. Use method 2 if the volume of the mixing container is not known. Each of these methods is described below.
A.2.2.1 Method 1 (Known mixing container volume)
From the "per volume solution" column of Table 7, determine the determine the weight in kg (or lb) of dry salt required to make 1 m3 (or 1 gal) of solution at the desired concentration level. This value, multiplied by the volume of the container, gives the total weight of dry salt required.
Fill the container approximately 2/3 full of water, then add the required dry salt gradually while stirring with a paddle by hand, with a mechanical agitator, or with an air bubbler.
After the salt has dissolved, add water to the container to bring the level to the working volume and then agitate the solution slowly until a uniform mixture is obtained. Some insoluble precipitate from the impurities will remain in the bottom of the tank until physically removed.
Finally, the salt brine should be tested with a hydrometer to determine the percentage of concentration that has been produced. For anti-icing operations, the concentration of the salt brine should be as close to 23.3 percent as possible, but no more than 25 percent. If the concentration is more than 25 percent, some water should be added to the mixture to reduce the concentration to the desired level. If the concentration is less than 23 percent some salt should be added to the mixture to raise the concentration to the desired level. The table of hydrometer readings versus percent concentration of salt given in Section 3.1.2.2 can be used for these tests.
Table 7. Proportions for preparing sodium chloride solutions from commercial grade salt (i.e., up to 5 percent impurities).
|
% NaCl actual |
Weight NaCl |
Crystallization |
Weight per unit | |
|
per volume solution kg/m3 (lb/gal) |
per volume water kg/m3 (lb/gal) |
temperature
|
volume of solution
| |
|
10 |
108 (0.9) |
96 (0.8) |
-6.7 (20) |
1072 (8.95) |
|
15 |
168 (1.4) |
156 (1.3) |
-11.1 (12) |
1112 (9.28) |
|
20 |
228 (1.9) |
204 (1.7) |
-17.8 (0) |
1150 (9.6) |
|
23* |
276 (2.3) |
228 (1.9) |
-21.1 (-6) |
1169 (9.76) |
|
25 |
300 (2.5) |
252 (2.1) |
-8.9 (16) |
1234 (10.3) |
*Note. This is the approximate eutectic composition (see explanation in calcium chloride entry above).
A.2.2.2 Method 2 (Unknown mixing container volume)
Fill a hopper tank with dry salt and let water slowly run through the salt by gravity action to the bottom of the tank.
Discharge the brine at the bottom of the hopper tank into a holding tank below the hopper tank. Pump the brine from the holding tank into a larger storage tank. Stir the mixture in the larger storage tank with a mechanical agitator or with an air bubbler.
Continue to add salt and water to the hopper tank until the large storage tank is almost full.
Determine the percentage of concentration of the salt brine using a hydrometer and the table in Section 3.1.2.2. Method 2 will produce up to a 100 percent saturated brine solution depending on the rate of water flow through the salt. A 100 percent saturated brine solution corresponds to a 27 percent concentration level. Water should be added now to the storage tank to reduce the concentration of the mixture to the 23 to 25 percent level.
Finally, the required number of pounds of salt used in Method 2 can be roughly calculated by multiplying the total volume of water by the weight of sodium chloride per volume of water given in the "per volume water" column of Table 7 that corresponds to the percent concentration level achieved. This estimate of the amount of salt used can be used to generate subsequent volumes of mixture to fill the storage tank when it is completely empty.
Method 2 above has been used successfully by several States to generate a salt brine for anti-icing operations. In practice, the brine generation process operates continuously during a storm. This is because liquid brine is drawn from the storage tank throughout the storm to fill the tanks on the spreader vehicles. In this situation, it is important to frequently monitor the percentage concentration level of the mixture in the storage tank. Adjustments should be made to mixture when necessary to achieve the desired level of concentration.
A.3 MAGNESIUM CHLORIDE, MgCl2
The principal source of this ice control chemical is brines from Great Salt Lake. Though it is available in solid (flake) form, it is used in liquid form for ice control. Eutectic temperature is about -33oC (-28oF) at a concentration of 21.6 percent. Its ice melting capacity is about 40 percent greater than CaCl2. Proprietary mixtures are available containing 20 to 25 percent MgCl2 with various corrosion inhibitor additives. One proprietary compound reportedly has an eutectic temperature of -20oC (-4oF). These solutions are effective ice-melting agents at temperatures above -7oC (19oF).
A.4 CALCIUM MAGNESIUM ACETATE, [CaMg2 (CH3COO)2]6
Currently there is only one commercial source for CMA, using the reaction of acetic acid with dolomitic limestone for production. The acetic acid, the costly component of the compound, is manufactured from natural gas or petroleum, though small quantities have been produced by biodegradation of agricultural wastes. The compound is available as pellets. Though not as soluble in water as NaCl and CaCl2, solutions can be made at point of use for use as a prewetting agent or straight chemical application. It is not a highly effective deicing chemical in solid form because of its affinity for water and its light particle mass. Its benefit is that snow is made mealy and will not compact. CMA is primarily a mixture of calcium and magnesium acetates, produced with a 3/7 Ca/Mg ratio which was found to be optimum in previous FHWA studies. The eutectic temperature is about -28oC (-18oF) at a concentration of 32.5 percent.
A.4.2 Preparation of liquid CMA
Liquid CMA is prepared by dissolving pelletized CMA in water. This process results in a murky solution that settles in time to produce a clear liquid CMA on top and discardable insolubles on the bottom. A 25 percent concentration of liquid CMA is recommended for anti-icing operations. Specifics of liquid CMA production are given in Section 3.1.2.2.
A.5 POTASSIUM ACETATE, KC2H3O2
Potassium acetate, or KAc as it is commonly known, is produced by the reaction of acetic acid with potassium carbonate. The sources of acetic acid are the same as are used in the production of CMA. Potassium carbonate is one of the group of salts commercially known as potash. Potassium carbonate was originally obtained by running water through wood ashes and boiling the resulting solution in large iron pots. The substance that formed was called potash. Potassium carbonate is currently produced by one of several processes that use potassium chloride, another salt of the potash family. The compound, potassium acetate, is a white, crystalline, deliquescent powder that has a saline taste. It is soluble in water and alcohol. Solutions are alkaline under a litmus test. The dry compound is combustible but is used as a dehydrating agent, a reagent in analytical chemistry, and in the production of synthetic flavors, in addition to other uses. The eutectic temperature of a KAc and water solution is -60oC (-76oF) at a concentration of 49 percent. A commercial form of liquid KAc, containing a 50 percent concentration by weight plus corrosion inhibitors, has been used as a prewetting agent with dry salt or as a straight chemical application. Some experience has been gained with the straight liquid form during anti-icing experiments (1).
In order for a chemical to act as a freezing-point depressant, it must go into solution. A solid chemical applied as an anti-icing treatment must cover the highway pavement surface as rapidly as possible in solution form to act as a barrier to the formation of a bonded snow or ice layer anywhere on the road. The dissolution of two common chemicals used in snow and ice control operations, sodium chloride (salt) and calcium chloride, will be used as examples to help explain the brine generation process.
Energy is required to initiate the solution process and to continue it. The solution process, in the case of salt, will take place very slowly. A dry particle of salt placed on a dry surface will just sit there for a time until it can absorb enough thermal energy from the surrounding environment to a point where a liquid film is formed on the surface of the particle. This initial brine then triggers the solution of the rest of the salt. As the particle dissolves, it continues to absorb thermal energy from its surroundings. This type of absorption process is called an endothermic reaction.
The rate at which salt goes into solution can be accelerated by several means. It can find free moisture or liquid on the pavement surface to start the brine generation process. Or, a liquid can be added to the surface of the salt particles before they are placed on the pavement surface. This second means of applying a liquid to dry salt is accomplished by a prewetting process.
The solution process of calcium chloride takes place much faster than that of salt. This is because calcium chloride is both hygroscopic and deliquescent (CaCl2 will absorb moisture at a relative humidity (RH) of 42 percent and higher; NaCl will not begin to absorb moisture until a RH of 76 percent is reached). Thus, solid calcium chloride will absorb moisture from the air until it dissolves. The brine solution will continue to absorb moisture until an equilibrium is reached between the vapor pressure of the solution and that of the air. If the humidity of the air increases, more moisture is absorbed by the solution. If the humidity of the air decreases, water evaporates from the solution to the air. As the particle of calcium chloride dissolves, it releases a considerable amount of heat. This type of process is called an exothermic reaction.
When calcium chloride and salt are combined, they complement each other as snow and ice control chemicals. When combined, the deliquescent calcium chloride absorbs moisture from its surroundings releasing heat and thereby increasing the rate of solution of sodium chloride. These reactions produce brine quickly which sustains the continued brine generation of the two chemicals.
The solubility of all chemicals varies with temperature. The lower the temperature, the less the solubility. This decrease in solubility has a limit, a point where no more of the chemical can dissolve and depress the freezing-point.
The freezing-point of a brine can best be described by reference to the phase diagram of a generic salt-water solution shown in the small plot inserted in the lower left of Figure 16. The solid curve is a plot of concentration of a salt (X-axis) versus temperature (Y-axis). The solid curve separates the phases of the solution. Above this curve, the salt is totally in solution. The lowest temperature on the curve is called the temperature at the eutectic point, or eutectic temperature. Below this temperature (and below the dashed line), no solution exists, only a mixture of ice and solid salt. A mixture of ice and salt solution exists to the left of the solid curve and above the dashed line. A mixture of solid salt and salt solution exists to the right of the solid curve and above the dashed line. Thus, the solid curve describes the freezing-point of a brine as a function of the concentration of the salt solution.
Some comments are given below about the important characteristics of the generic phase diagram.
The freezing-point of the brine solution decreases with increasing concentration up to the eutectic composition. The freezing-point of the brine solution will increase as the concentration increases beyond the eutectic composition.
Brine solutions having a concentration less than the eutectic composition have a freezing-point lower than the melting temperature of pure ice or 0oC (32oF).
In snow and ice control operations and particularly during anti-icing treatments, it is necessary to operate with brine solutions as close as possible to, but less than, the eutectic composition. The brine solution concentration will decrease as it is diluted with water from either the melting of snow/ice or falling rain/freezing rain. Consequently, it is important to monitor the dilution process so that the solution concentration does not decrease to a value which corresponds to a temperature in the freezing temperature range above the pavement temperature. When this occurs, a refreeze of the solution will take place.
B.2 NaCl AND CaCl2
The phase diagrams of NaCl and CaCl2 solutions are also presented in Figure 16. As can be seen, the eutectic temperature of the calcium chloride-water system is lower than the eutectic temperature of the sodium chloride-water system. The eutectic composition of the calcium chloride-water system is approximately 30 percent CaCl2 and 70 percent H20 by weight which remains a solution as low as -51oC (-60oF). The eutectic composition of the sodium chloride (common road salt)-water system is 23 percent NaCl and 77 percent H20 by weight, which freezes at about -21oC (-6oF).
The 30 percent concentration of calcium chloride shown in Figure 16 at the eutectic temperature is higher than the corresponding concentration of a commercially available pelletized form of calcium chloride (29.6 to 29.8 percent). Some differences can occur between individual phase diagrams of commercially available calcium chloride-water systems because of the presence and amounts of other chemical elements.
Some evidence suggest that anti-icing operations should not be conducted (using liquid, prewetted, or dry salt) when the pavement temperature is at or below about -9.5oC (15oF) (2). Some highway agencies also believe that it is not practical to use salt below -9oC (15oF) for general snow and ice control operations, at least not without calcium chloride. This experience has convinced them that salt’s action is too slow at these lower temperatures. An inspection of Figure 16 shows that the phase diagrams of NaCl and CaCl2 are not too dissimilar in the temperature range of 0oC (32oF) down to -10oC (15oF) or even down to about -15oC (5oF). Thus, the two chemical brines have about the same solidification (freezing) characteristics in this temperature range. The fact that calcium chloride has a much lower eutectic temperature than sodium chloride is not of importance for anti-icing operations.
B.3 MgCl2
The phase diagram of magnesium chloride, MgCl2, solutions is presented in Figure 17 together with those of CMA, KAc, and the chloride solutions discussed above. The eutectic temperature of the magnesium chloride-water system is between that of NaCl and CaCl2. The eutectic composition for the magnesium chloride-water system in this figure is 21.6 percent MgCl2 and 78.4 percent H20 by weight which freezes at about -33oC (-28oF). The density and chemical composition of MgCl2 brines can vary somewhat with the source of MgCl2 and with seasonal weather fluctuations which affect the solar evaporation process used in the production of flake MgCl2. The chemical composition of MgCl2 brines can include, in addition to magnesium and chloride, such components as sulfates, sodium, potassium, lithium, bromine, and iron. Consequently, some differences can occur between individual phase diagrams of commercially available magnesium chloride-water systems.
It is necessary to check the percent concentration of MgCl2 brines before use in anti-icing treatments either as a prewetting agent for a solid chemical or as a straight liquid. It is important that the percent concentration not be too high above that at the eutectic temperature. If it is, there is the possibility that the solution might clog the spreader spray nozzles and/or burn out electric pumps.
An interesting comparison of the freezing-point of the three chloride solutions can be made by reference to Figure 17. For a given pavement temperature below 0oC (32oF) but above the eutectic temperature of NaCl, a MgCl2 solution will refreeze at a lower concentration than the corresponding concentrations of either CaCl2 or NaCl at that temperature. For example, the refreeze concentration of MgCl2 at -10oC (15oF) is about 11 percent while the refreeze concentrations of CaCl2 and NaCl at that temperature are about 12.5 percent and 13.5 percent, respectively. This means that MgCl2 brines can be diluted more than CaCl2 and NaCl before refreezing at a given temperature. However, once the dilution process starts, the refreeze temperature of MgCl2 rises faster than do the refreeze temperatures of both CaCl2 and NaCl. The slope of the MgCl2 curve to the left of its eutectic concentration is steeper than the slope of either the CaCl2 or NaCl curves until the three brine concentrations reach about 5 percent or a corresponding temperature of about -3oC (27oF). Between this temperature and 0oC (32oF), all three brines have about the same refreeze characteristics.
One final comment about the chloride solution curves in Figure 17. The refreeze temperature of NaCl solutions rises slower with dilution than do the refreeze temperatures of either CaCl2 or MgCl2. However, a slightly higher concentration of a NaCl solution (slightly more salt) is needed than the corresponding concentration of CaCl2 at a given temperature to keep the two brine solutions from refreezing. A much higher concentration of NaCl is needed than the corresponding concentration of MgCl2 at the same temperature to keep the two brine solutions from refreezing.
B.4 CMA AND KAc
The curve for CMA (Figure 17) was determined from different percent concentration solutions made by dissolving commercially available CMA supplied in a dry pellet form. The curve for KAc was determined using a commercially available liquid form of KAc. The eutectic temperature for the CMA-water system in Figure 17 is -27.5oC (-17.5oF) at a concentration of 32.5 percent. The eutectic temperature for the KAc-water system is -60oC (-76oF) at a concentration of 49 percent. The curves for CMA and KAc almost coincide with each other. Also, they have a much flatter slope than the other three curves. This is an important feature of both CMA and KAc solutions. The refreeze temperature of both CMA and KAc solutions rises slower with dilution than do the refreeze temperatures of either NaCl, CaCl2, or MgCl2. This feature makes them well suited for being used in a liquid form during anti-icing treatments. This is especially true for their use in a liquid form for the pretreatment of bridge decks in anticipation of frosting, or localized icing conditions.
All is not favorable with the use of CMA or KAc over that of, say, NaCl. A much higher concentration of either CMA or KAc solution is needed than the corresponding concentration of NaCl at a given temperature to keep the solutions from refreezing. The solution concentration of CMA must be 1.41 times higher than the NaC1 solution concentration at -9.4oC (15oF) (19 percent for CMA versus 13.5 percent for NaCl) to keep both solutions from refreezing. This factor increases to 1.54 at -3.9oC (25oF). The solution concentration of KAc must be about 1.37 and 1.38 higher than the NaCl solution concentrations at -9.4oC (15oF) and -3.9oC (25oF), respectively. These differences in concentrations needed for both CMA and KAc translate into considerably higher costs per application treatment for both chemicals than NaCl.
This appendix is a guide to highway anti-icing operations for maintenance field personnel. Its purpose is to suggest maintenance actions for preventing the formation or development of packed and bonded snow or bonded ice during a variety of winter weather events. It is intended to complement the decision-making and management practices of a systematic anti-icing program so that roads can be efficiently maintained in the best possible condition.
The guidance is based upon the results of four years of anti-icing field testing conducted by 15 State highway agencies and supported by the Strategic Highway Research Program (SHRP) and the Federal Highway Administration (FHWA). It has been augmented with practices developed outside the U.S., where necessary, for completeness. The recommendations are subject to refinement as U.S. highway agencies gain additional experience with anti-icing operations. Final decisions for their implementation rests with management personnel.
C.2 GUIDANCE FOR ANTI-ICING OPERATIONS
Guidance for anti-icing operations is presented in Tables 8 to 13 for six distinctive winter weather events. The six events are:
Light Snow Storm
Light Snow Storm with Period(s) of Moderate or Heavy Snow
Moderate or Heavy Snow Storm
Frost or Black Ice
Freezing Rain Storm
Sleet Storm
The tables suggest the appropriate maintenance action to take during an initial or subsequent (follow-up) anti-icing operation for a given precipitation or icing event. Each action is defined for a range of pavement temperatures and an associated temperature trend. For some events the operation is dependent not only on the pavement temperature and trend, but also upon the pavement surface or the traffic condition at the time of the action. Most of the maintenance actions involve the application of a chemical in either a dry solid, liquid, or prewetted solid form. Application rates ("spread rates") are given for each chemical form where appropriate. These are suggested values and should be adjusted, if necessary to achieve increased effectiveness or efficiency, for local conditions. The rates given for liquid chemicals are the equivalent dry chemical rates. Application rates in volumetric units such as L/lane-km (or gal/lane-mi) must be calculated from these dry chemical rates for each chemical and concentration.
Comments and notes are given in each table where appropriate to further guide the maintenance field personnel in their anti-icing operations.
C.3 GLOSSARY OF TERMS
Black ice. Popular term for a very thin coating of clear, bubble-free, homogeneous ice which forms on a pavement with a temperature at or slightly above 0oC (32oF) when the temperature of the air in contact with the ground is below the freezing-point of water and small slightly supercooled water droplets deposit on the surface and coalesce (flow together) before freezing.
Dry chemical spread rate. The chemical application rate. For solid applications it is simply the weight of the chemical applied per lane kilometer (or mile). For liquid applications it is the weight of the dry chemical in solution applied per lane kilometer (or mile).
Freezing rain. Supercooled droplets of liquid precipitation falling on a surface whose temperature is below or slightly above freezing, resulting in a hard, slick, generally thick coating of ice commonly called glaze or clear ice. Non-supercooled raindrops falling on a surface whose temperature is well below freezing will also result in glaze.
Frost. Also called hoarfrost. Ice crystals in the form of scales, needles, feathers or fans deposited on surfaces cooled by radiation or by other processes. The deposit may be composed of drops of dew frozen after deposition and of ice formed directly from water vapor at a temperature below 0oC (32oF) (sublimation).
Light snow. Snow falling at the rate of less than 12 mm (1/2 in) per hour; visibility is not affected adversely.
Liquid chemical. A chemical solution; the weight of the dry chemical in solution applied per lane kilometer (or mile) is the chemical application rate – the "dry chemical spread rate" – used in this appendix.
Moderate or heavy snow. Snow falling at a rate of 12 mm (1/2 in) per hour or greater; visibility may be reduced.
Sleet. A mixture of rain and of snow which has been partially melted by falling through an atmosphere with a temperature slightly above freezing.
Slush. Accumulation of snow which lies on an impervious base and is saturated with water in excess of its freely drained capacity. It will not support any weight when stepped or driven on but will "squish" until the base support is reached.
Table 8. Weather event: light snow storm.
|
PAVEMENT |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | |||||
|
TEMPERATURE RANGE,
|
pavement surface at time of |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) | |||
|
initial operation |
liquid |
solid or prewetted solid |
liquid |
solid or prewetted solid | ||||
|
Above 0oC (32oF), steady or rising |
Dry, wet, slush, or light snow cover |
None, see comments |
None, see comments |
1) Monitor pavement temperature closely for drops toward 0oC (32oF) and below 2) Treat icy patches if needed with chemical at
| ||||
|
Above 0oC (32oF), 0oC (32oF) or below is imminent; |
Dry |
Apply liquid or prewetted solid chemical |
28
|
28
|
Plow as needed; reapply liquid or solid chemical when needed |
28
|
28
|
1) Applications will need to be more frequent at lower temperatures and higher snowfall rates 2) It is not advisable to apply a liquid chemical at the indicated spread rate when the pavement |
|
ALSO -7 to 0oC (20 to 32oF), remaining in range |
Wet, slush, or light snow cover |
Apply liquid or solid chemical |
28
|
28
|
temperature drops below -5oC (23oF) 3) Do not apply liquid chemical onto heavy snow accumulation or packed snow | |||
|
-10 to -7oC (15 to 20oF), remaining in range |
Dry, wet, slush, or light snow cover |
Apply prewetted solid chemical |
55
|
Plow as needed; reapply prewetted solid chemical when needed |
55
|
If sufficient moisture is present, solid chemical without prewetting can be applied | ||
|
Below -10oC (15oF), steady or falling |
Dry or light snow cover |
Plow as needed |
Plow as needed |
1) It is not recommended that chemicals be applied in this temperature range 2) Abrasives can be applied to enhance traction | ||||
Notes
CHEMICAL APPLICATIONS. (1) Time initial and subsequent chemical applications to prevent deteriorating conditions or development of packed and bonded snow. (2) Apply chemical ahead of traffic rush periods occurring during storm.
PLOWING. If needed, plow before chemical applications so that excess snow, slush, or ice is removed and pavement is wet, slushy, or lightly snow covered when treated.
Table 9. Weather event: light snow storm with period(s) of moderate or heavy snow.
|
PAVEMENT |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | |||||||
|
TEMPERATURE RANGE,
|
pavement surface at time of |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
dry chemical spread
| |||||
|
initial operation |
liquid |
solid or prewetted |
liquid |
solid or prewetted solid | ||||||
|
solid |
light snow |
heavier snow |
light snow |
heavier snow | ||||||
|
Above 0oC (32oF), steady or rising |
Dry, wet, slush, or light snow cover |
None, see comments |
None, see comments |
1) Monitor pavement temperature closely for drops toward 0oC (32oF) and below 2) Treat icy patches if needed with chemical at 28 kg/lane-km
| ||||||
|
Above 0oC (32oF), 0oC (32oF) or below is imminent; |
Dry |
Apply liquid or prewetted solid chemical |
28
|
28
|
Plow as needed; reapply liquid or solid |
28
|
55
|
28
|
55
|
1) Applications will need to be more frequent at lower temperatures and higher snowfall rates 2) Do not apply liquid chemical onto |
|
ALSO -4 to 0oC (25 to 32oF), remaining in range |
Wet, slush, or light snow cover |
Apply liquid or solid chemical |
28
|
28
|
chemical when needed |
heavy snow accumulation or packed snow 3) After heavier snow periods and during light snow fall, reduce chemical rate to
| ||||
|
-10 to -4oC (15 to 25oF), remaining in range |
Dry, wet, slush, or light snow cover |
Apply prewetted solid chemical |
55
|
Plow as needed; reapply prewetted solid chemical when needed |
55
|
70
|
1) If sufficient moisture is present, solid chemical without prewetting can be applied 2) Reduce chemical rate to 55 kg/lane-km (200 lb/lane-mi) after heavier snow periods and during light snow fall; continue to plow and apply chemicals as needed | |||
|
Below -10oC (15oF), steady or falling |
Dry or light snow cover |
Plow as needed |
Plow as needed |
1) It is not recommended that chemicals be applied in this temperature range 2) Abrasives can be applied to enhance traction | ||||||
Notes
CHEMICAL APPLICATIONS. (1) Time initial and subsequent chemical applications to prevent deteriorating conditions or development of packed and bonded snow. (2) Anticipate increases in snowfall intensity. Apply higher rate treatments prior to or at the beginning of heavier snowfall periods to prevent development of packed and bonded snow. (3) Apply chemical ahead of traffic rush periods occurring during storm.
PLOWING. If needed, plow before chemical applications so that excess snow, slush, or ice is removed and pavement is wet, slushy, or lightly snow covered when treated.
Table 10. Weather event: moderate or heavy snow storm.
|
PAVEMENT |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | |||||
|
TEMPERATURE RANGE,
|
pavement surface at time of |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) | |||
|
initial operation |
liquid |
solid or prewetted solid |
liquid |
solid or prewetted solid | ||||
|
Above 0oC (32oF), steady or rising |
Dry, wet, slush, or light snow cover |
None, see comments |
None, see comments |
1) Monitor pavement temperature closely for drops toward 0oC (32oF) and below 2) Treat icy patches if needed with chemical at
| ||||
|
Above 0oC (32oF), 0oC (32oF) or below is imminent; |
Dry |
Apply liquid or prewetted solid chemical |
28
|
28
|
Plow accumulation and reapply liquid or solid |
28
|
28
|
1) If the desired plowing/treatment frequency cannot be maintained, the spread rate can be increased to
|
|
ALSO -1 to 0oC (30 to 32oF), remaining in range |
Wet, slush, or light snow cover |
Apply liquid or solid chemical |
28
|
28
|
chemical as needed |
2) Do not apply liquid chemical onto heavy snow accumulation or packed snow | ||
|
-4 to -1oC (25 to 30oF), remaining in range |
Dry |
Apply liquid or prewetted solid chemical |
55
|
42-55
|
Plow accumulation and reapply |
55
|
55
|
1) If the desired plowing/treatment frequency cannot be maintained, the spread rate can be increased to
|
|
Wet, slush, or light snow cover |
Apply liquid or solid chemical |
55
|
42-55
|
liquid or solid chemical as needed |
operational cycles 2) Do not apply liquid chemical onto heavy snow accumulation or packed snow | |||
|
-10 to -4oC (15 to 25oF), remaining in range |
Dry, wet, slush, or light snow cover |
Apply prewetted solid chemical |
55
|
Plow accumulation and reapply prewetted solid chemical as needed |
70
|
1) If the desired plowing/treatment frequency cannot be maintained, the spread rate can be increased to
2) If sufficient moisture is present, solid chemical without prewetting can be applied | ||
|
Below -10oC (15oF), steady or falling |
Dry or light snow cover |
Plow as needed |
Plow accumulation as needed |
1) It is not recommended that chemicals be applied in this temperature range 2) Abrasives can be applied to enhance traction | ||||
Notes
CHEMICAL APPLICATIONS. (1) Time initial and subsequent chemical applications to prevent deteriorating conditions or development of packed and bonded snow -- timing and frequency of subsequent applications will be determined primarily by plowing requirements. (2) Apply chemical ahead of traffic rush periods occurring during storm.
PLOWING. Plow before chemical applications so that excess snow, slush, or ice is removed and pavement is wet, slushy, or lightly snow covered when treated.
Table 11. Weather event: frost or black ice.
|
PAVEMENT |
TRAFFIC |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | ||||
|
TEMPERATURE RANGE,
|
CONDITION |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
dry chemical spread rate, kg/lane-km (lb/lane-mi) | |||
|
RELATION TO DEW POINT |
liquid |
solid or prewetted solid |
liquid |
solid or prewetted solid | ||||
|
Above 0oC (32oF), steady or rising |
Any level |
None, see comments |
None, see comments |
Monitor pavement temperature closely; begin treatment if temperature starts to fall to 0oC (32oF) or below and is at or below dew point | ||||
|
-2 to 2oC (28 to 35oF), remaining in range or falling to 0oC |
Traffic rate less than 100 vehicles per h |
Apply prewetted solid chemical |
7-18
|
Reapply prewetted solid chemical as needed |
7-18
|
1) Monitor pavement closely; if pavement becomes wet or if thin ice forms, reapply chemical at higher indicated rate 2) Do not apply liquid chemical on ice so thick | ||
|
(32oF) or below, and equal to or below dew point |
Traffic rate greater than 100 vehicles per h |
Apply liquid or prewetted solid chemical |
7-18
|
7-18
|
Reapply liquid or prewetted solid chemical as needed |
11-32
|
7-18
|
that the pavement can not be seen |
|
-7 to -2oC (20 to 28oF), remaining in range, and equal to or below dew point |
Any level |
Apply liquid or prewetted solid chemical |
18-36
|
18-36
|
Reapply liquid or prewetted solid chemical when needed |
18-36
|
18-36
|
1) Monitor pavement closely; if thin ice forms, reapply chemical at higher indicated rate 2) Applications will need to be more frequent at higher levels of condensation; if traffic volumes are not enough to disperse condensation, it may be necessary to increase frequency 3) It is not advisable to apply a liquid chemical at the indicated spread rate when the pavement temperature drops below -5oC (23oF) |
|
-10 to -7oC (15 to 20oF), remaining in range, and equal to or below dew point |
Any level |
Apply prewetted solid chemical |
36-55
|
Reapply prewetted solid chemical when needed |
36-55
|
1) Monitor pavement closely; if thin ice forms, reapply chemical at higher indicated rate 2) Applications will need to be more frequent at higher levels of condensation; if traffic volumes are not enough to disperse condensation, it may be necessary to increase frequency | ||
|
Below -10oC (15oF), steady or falling |
Any level |
Apply abrasives |
Apply abrasives as needed |
It is not recommended that chemicals be applied in this temperature range | ||||
Notes
TIMING. (1) Conduct initial operation in advance of freezing. Apply liquid chemical up to 3 h in advance. Use longer advance times in this range to effect drying when traffic volume is low. Apply prewetted solid 1 to 2 h in advance. (2) In the absence of precipitation, liquid chemical at 21 kg/lane-km (75 lb/lane-mi) has been successful in preventing bridge deck icing when placed up to 4 days before freezing on higher volume roads and 7 days before on lower volume roads.
Table 12. Weather event: freezing rain storm.
|
PAVEMENT |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | ||
|
TEMPERATURE RANGE,
|
maintenance action |
chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
chemical spread rate,
| |
|
Above 0oC (32oF), steady or rising |
None, see comments |
None, see comments |
1) Monitor pavement temperature closely for drops toward 0oC (32oF) and below 2) Treat icy patches if needed with prewetted solid chemical at 21-28 kg/lane-km (75-100 lb/lane-mi) | ||
|
Above 0oC (32oF), 0oC (32oF) or below is imminent |
Apply prewetted solid chemical |
21-28 (75-100) |
Reapply prewetted solid chemical as needed |
21-28 (75-100) |
Monitor pavement temperature and precipitation closely |
|
-7 to 0oC (20 to 32oF), remaining in range |
Apply prewetted solid chemical |
21-70
|
Reapply prewetted solid chemical as needed |
21-70
|
1) Monitor pavement temperature and precipitation closely 2) Increase spread rate toward higher indicated rate with decrease in pavement temperature or increase in intensity of freezing rainfall 3) Decrease spread rate toward lower indicated rate with increase in pavement temperature or decrease in intensity of freezing rainfall |
|
-10 to -7oC (15 to 20oF), remaining in range |
Apply prewetted solid chemical |
70-110
|
Reapply prewetted solid chemical as needed |
70-110
|
1) Monitor precipitation closely 2) Increase spread rate toward higher indicated rate with increase in intensity of freezing rainfall 3) Decrease spread rate toward lower indicated rate with decrease in intensity of freezing rainfall |
|
Below -10oC (15oF), steady or falling |
Apply abrasives |
Apply abrasives as needed |
It is not recommended that chemicals be applied in this temperature range | ||
Notes
CHEMICAL APPLICATIONS. (1) Time initial and subsequent chemical applications to prevent glaze ice conditions. (2) Apply chemical ahead of traffic rush periods occurring during storm.
Table 13. Weather event: sleet storm.
|
PAVEMENT |
INITIAL OPERATION |
SUBSEQUENT OPERATIONS |
COMMENTS | ||
|
TEMPERATURE RANGE,
|
maintenance action |
chemical spread rate, kg/lane-km (lb/lane-mi) |
maintenance action |
chemical spread rate,
| |
|
Above 0oC (32oF), steady or rising |
None, see comments |
None, see comments |
1) Monitor pavement temperature closely for drops toward 0oC (32oF) and below 2) Treat icy patches if needed with prewetted solid chemical at 35 kg/lane-km (125 lb/lane-mi) | ||
|
Above 0oC (32oF), 0oC (32oF) or below is imminent |
Apply prewetted solid chemical |
35
|
Plow as needed, reapply prewetted solid chemical when needed |
35
|
Monitor pavement temperature and precipitation closely |
|
-2 to 0oC (28 to 32oF), remaining in range |
Apply prewetted solid chemical |
35-90
|
Plow as needed, reapply prewetted solid chemical when needed |
35-90
|
1) Monitor pavement temperature and precipitation closely 2) Increase spread rate toward higher indicated rate with increase in sleet intensity 3) Decrease spread rate toward lower indicated rate with decrease in sleet intensity |
|
-10 to -2oC (15 to 28oF), remaining in range |
Apply prewetted solid chemical |
70-110
|
Plow as needed, reapply prewetted solid chemical when needed |
70-110
|
1) Monitor precipitation closely 2) Increase spread rate toward higher indicated rate with decrease in pavement temperature or increase in sleet intensity 3) Decrease spread rate toward lower indicated rate with increase in pavement temperature or decrease in sleet intensity |
|
Below -10oC (15oF), steady or falling |
Plow as needed |
Plow as needed |
1) It is not recommended that chemicals be applied in this temperature range 2) Abrasives can be applied to enhance traction | ||
Notes
CHEMICAL APPLICATIONS. (1) Time initial and subsequent chemical applications to prevent the sleet from bonding to the pavement. (2) Apply chemical ahead of traffic rush periods occurring during storm.
1. U.S. Army Cold Regions Research and Engineering Laboratory. FHWA Test and Evaluation Project No. 28, "Anti-Icing Technology," Field Evaluation Report. Hanover, New Hampshire, 1996.
2. Blackburn R.R., E.J. McGrane, C.C. Chappelow, D.W. Harwood, and E. J. Fleege. Development of Anti-Icing Technology. Report SHRP-H-385, National Research Council, Washington, DC, 1994.
3. The Snowfighter’s Handbook. The Salt Institute, Alexandria, Virginia, 1991.
4. Boselly, S.E. and D. Ernst. Road Weather Information Systems, Volume 2: Implementation Guide. Report SHRP-H-351, SHRP, National Research council, Washington, DC, 1993.
5. Boselly, S.E., J.E. Thornes, C. Ulberg, and D. Ernst. Road Weather Information Systems, Volume 1: Research Report. Report SHRP-H-350, SHRP, National Research Council, Washington, DC, 1993.
6. SHRP Snow and Ice Control Showcasing and Implementation, FHWA Contract No. DTFH61-94-C-00177, Federal Highway Administration, Washington, DC.