U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-07-043
Date: July 2007 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chapter 2. Experimental WorkCORROSION PROTECTION SYSTEMSThis study involves the evaluation of 11 systems in which epoxy-coated reinforcement is combined with another corrosion protection system. The research includes seven bar types, one uncoated and six with a fusion-bonded epoxy coating. Uncoated conventional reinforcing steel and conventional epoxy-coated reinforcement serve as the controls. The multiple corrosion protection systems include conventional epoxy-coated reinforcement used in conjunction with one of three corrosion inhibitors, bars that are treated with a primer coating containing microencapsulated calcium nitrite (a corrosion inhibitor) prior to coating with conventional epoxy, bars with improved adhesion between the epoxy and the reinforcing steel, obtained through the use of either a zinc chromate pretreatment or special epoxies with higher adhesion, the combination of bars with an improved adhesion epoxy and the addition of calcium nitrite to the mortar or concrete used in the tests, and bars with multiple coatings, consisting of a 50- micrometers (µm) (2-milli-inch (mil)) layer of 98 percent zinc–2 percent aluminum that is, in turn, coated with a conventional epoxy. The systems are listed in table 1, along with the shorthand notation that will be used in this report, and are described next. Control SystemsUncoated conventional steel (Conv.)—All tests of both uncoated and coated bars involve the use of a single heat of No. 16 (No. 5) Grade 420 (60) A 615(8) reinforcing steel. The chemical analysis of the steel is given in table 2. Conventional epoxy-coated reinforcement (ECR)—The conventional fusion-bonded thermoset epoxy-coated reinforcement used as the control is coated with Scotchkote™ 413 manufactured by 3M Corporation. Epoxies With Increased AdhesionA number of techniques have been used to improve the adhesion of epoxy coatings to steel. Three systems are evaluated in this study. The first involves pretreatment of uncoated steel with zinc chromate prior to the application of the epoxy coating to improve adhesion. The procedure is used in Canada for all epoxy-coated reinforcement, but because it involves the use of hexavalent chromate, which represents a significant environmental problem, it is not widely used in the United States. As an alternative, DuPont and Valspar have developed epoxy powders with improved adhesion to reinforcing steel that do not require pretreating the bars. The three systems are identified, respectively, as ECR(Chromate), ECR(DuPont), and ECR(Valspar).
Corrosion InhibitorsThree corrosion inhibitors, one inorganic and two organic, are under study. Calcium nitrite [Ca(NO2)2] is the most widely used inorganic corrosion inhibitor in U.S. practice. Because calcium nitrite acts as a set-accelerating admixture, the form of the admixture containing a retarder, DCI-S, produced by W. R. Grace, is used in this study. The organic corrosion inhibitors are Rheocrete 222+, a water-based combination of amines and esters produced by BASF Admixtures, and Hycrete, a salt of alkenyl-substituted succinic acid, produced by Broadview Technologies. A fourth system is also evaluated in which an epoxy coating (Scotchkote 413) is applied to the bars after the application of a primer coating, also produced by 3M, that contains microencapsulated calcium nitrite. According to 3M, the latter system provides protection by releasing calcium nitrite as the epoxy coating is damaged. The systems are identified, respectively, as ECR(DCI), ECR(HY), ECR(RH), and ECR(primer/ Ca(NO2)2). Epoxies With Increased Adhesion Plus Ca(NO2)2The three types of epoxy-coated reinforcement with improved adhesion are also evaluated in mortar and concrete containing the corrosion inhibitor calcium nitrite. The systems are identified as ECR(Chromate)-DCI, ECR(DuPont)-DCI, and ECR(Valspar)-DCI. Multiple CoatingsWestern Coating has developed a patented process for multiple coated (MC) bars that involves the application of a layer of 98 percent zinc–2 percent aluminum to reinforcing steel prior to the application of the epoxy coating. The zinc layer has a nominal thickness of 50 µm (2 mils). Following application of the zinc, the bars in this study are coated with DuPont 8-2739 Flex West Blue, a conventional epoxy. One applicator applied the epoxy to the conventional ECR and ECR(Valspar) bars. A second applicator handled the MC and ECR(DuPont) bars, while two other applicators individually handled the ECR(Chromate) and ECR(primer/Ca(NO2)2) bars. PRETEST EVALUATION OF EPOXY-COATED BARSPrior to corrosion testing, the bars used in this study were evaluated for coating thickness and number of holidays. The bars were also evaluated for coating adhesion using the cathodic disbondment test, performed in accordance with ASTM A 775(9) and ASTM G 8.(10) The results of these tests are summarized in table 3.
Evaluation of Coating Thickness and HolidaysThe six types of epoxy-coated reinforcing bars used in this study were evaluated for coating thickness and number of holidays. The results are presented in table 3. All bars met the requirements of ASTM A 775(9) for coating thickness, with the exception of the bars with the calcium nitrite primer coating, which tended to have larger percentages of coating measurements below 175 µm (7 mils) and 125 µm (5 mils) than the maximum allowable values of 10 and 5 percent (ASTM A 775), respectively. Only the bars with the calcium nitrite primer coating exhibited holidays, although the number of holidays was below the maximum allowable of 3 holidays per meter (1 holiday per foot) specified in ASTM A 775. The other five bar types exhibited no measurable holidays on the full-size bars that were tested. Additional tests of small bar samples, however, indicated the presence of a small number of holidays on all bar types. Cathodic Disbondment TestsCathodic disbondment tests involve the penetration of the epoxy coating on a test specimen using a 3-millimeter (mm) (0.12-inch) diameter drill bit. The specimen is then immersed for 168 hours in an electrolyte (3 percent sodium chloride (NaCl) solution) at 24 ± 2 degrees Celsius (°C) (75 ± 3.6 degrees Fahrenheit (°F)) and maintained at a –1.5 volt (V) potential difference with an anode, measured with respect to a saturated calomel electrode (SCE). An examination is performed immediately upon termination of the test as follows: At the end of the test period, the test area is rinsed with warm tap water. The sample is immediately wiped dry, and the entire area of coating is visually examined at the edge of the intentional defect. A new defect, to serve as a reference, is then drilled in a portion of the coated area that was not immersed. Two radial cuts (at 90 degrees to each other, oriented 45 degrees with respect to the longitudinal axis of the bar) are made through the coating, intersecting the center of both intentional defects, using a sharp, thin-plated knife. An attempt is then made to lift the coating at both the reference defect and the submerged defect with the point of the knife. The bond at the reference defect is then used to judge the quality of the bond at the submerged holiday. Finally, the increase in radial area and total area of the disbonded coating at the submerged defect are measured and recorded. In this study, three rounds of cathodic disbondment tests were performed, with one specimen per round for each of the six types of epoxy-coated reinforcement. Tests were also performed on conventional epoxy-coated bars that had been used in a previous study. In accordance with ASTM A 775,(9) four radial measurements were taken of the disbonded region, at 0, 90, 180, and 270 degrees with respect to the longitudinal axis of the bar, and the values were averaged. The cathodic disbondment tests were recorded in terms of both the area of the disbonded coating (in accordance with ASTM G 8(10)) and the average coating disbondment radius (four measurements). The results are summarized in table 4. The area of the disbonded coating and the radius do not include the original penetration through the coating. As shown in table 4, the average coating disbondment radius was above 4 mm (the maximum allowed in Annex A1 of ASTM A 775) for the conventional ECR (5.9 mm) and the high adhesion Valspar [ECR(Valspar)] bars (4.9 mm), indicating that these bars failed the coating disbondment requirements. The multiple coated (MC) reinforcement (1.7 mm), high adhesion DuPont
[ECR(DuPont)] bars (2.8 mm), ECR with chromate pre-treatment [ECR(Chromate)] (1.0 mm), and ECR with the calcium nitrite primer [ECR(primer/Na(NO2)2)] (2.6 mm) met the coating disbondment requirements. Table 4 shows that the conventional epoxy-coated reinforcement exhibited the highest area of disbonded coating, with an average value of 178 mm2. The high adhesion Valspar bars had an area of disbonded coating of 151 mm2, followed by ECR with the calcium nitrite primer at 67 mm2 and the high adhesion DuPont bars at 65 mm2. The MC bars and ECR(Chromate) had the lowest areas of disbonded coating, with average values of 27 and 20 mm2, respectively. Like the conventional ECR used in this study, the conventional ECR from the previous study also exhibited an average disbondment radius, 5.5 mm, that exceeded the 4 mm allowed by ASTM A 775; the disbonded area equaled 168 mm2. The criteria in Annex A1 of A 775 are qualification requirements for the epoxy coating itself and are not meant to be applied to production bars, such as those used in this study. Thus, although all bars did not all meet the qualification criteria, they are all considered to be representative of bars that are used in practice. Further, as has been observed in earlier tests by McDonald et al.,(11) the performance of the bars in the cathodic disbondment tests has not proven to be a predictor of their performance in the corrosion tests in this study. CORROSION TEST PROCEDURESThe multiple corrosion protection systems are being evaluated using a combination of laboratory and field tests. The results of the laboratory tests, most of which are still ongoing, are presented in this report. The performance of each system is compared to that of conventional epoxy-coated reinforcement and uncoated mild steel reinforcement. The tests include rapid macrocell tests, bench-scale tests, and linear polarization resistance. Each of these techniques is discussed briefly. Rapid Macrocell TestsSummary of Method The response of the multiple corrosion protection systems was first evaluated using the rapid macrocell test, originally developed at the University of Kansas under the SHRP program(12,13) and since updated. (See references 14 through 21). The goal of the test is to obtain a realistic measure of the performance of corrosion protection systems in a short time period. The basic test specimen consists of either a bare reinforcing bar or a bar clad in mortar (mortar-wrapped), as illustrated in figures 1, 2, and 3. The contact surface between the mortar and the bar simulates the contact obtained between concrete and reinforcing bars in structures through the use of realistic water-cement and sand-cement ratios. The macrocell tests (figures 1 and 2) require two containers. The test specimen, either a bare or mortar-wrapped No. 16 (No. 5) bar, is placed in a 1.5-L (1.6-quart) container, along with simulated pore solution containing a preselected concentration [1.6 or 6.04 moles per kilogram of solvent (molal)-ion (4.68 or 15 percent) concentration] of sodium chloride. Two specimens Figure 1. Diagram. Schematic of macrocell test with bare bar specimens. 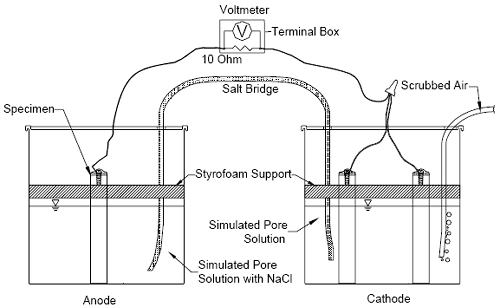 Figure 2. Diagram. Schematic of macrocell test with mortar-clad specimens. 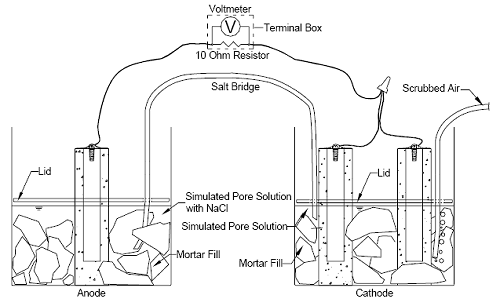 Figure 3. Diagram. Schematic of mortar-wrapped specimen containing a conventional reinforcing bar. 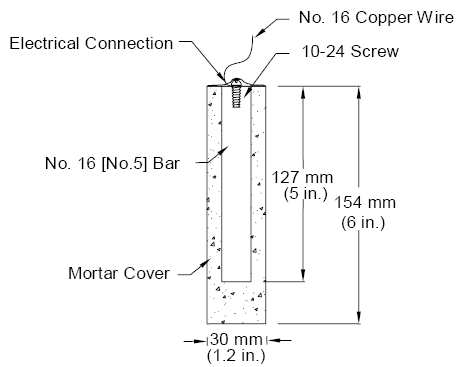 are placed in a second container and immersed in simulated pore solution (with no chlorides added). For mortar-wrapped specimens, crushed mortar fill is added to the containers to more closely simulate the concrete environment. The solution depth exposes 76 mm (3 inches) of a bar [including the 12.7 mm (0.5 inch) plastic cap used to protect the end of epoxy-coated bars] below the level of the solution. The two containers are connected by a salt bridge, and the test specimen in the pore solution containing sodium chloride (anode) is electrically connected across a single 10-ohm resistor to the two specimens in the simulated pore solution (cathode). The resistors are mounted between binding posts in a terminal box to consolidate the specimen wires. Air (scrubbed to remove CO2) is bubbled into the liquid surrounding the cathode to ensure an adequate supply of oxygen. Plastic lids are placed just above the surface of the solution to hold the specimens in place and reduce the evaporation of the solution. Holes are cut in the lids to introduce the specimens, a salt bridge, and the air supply. The air causes some evaporation, which is countered by adding deionized water to the container to maintain a constant volume of solution. The solutions in both containers are changed once every five weeks to further protect against carbonation.* The corrosion current and the rate of corrosion can be determined by measuring the voltage drop across the resistor. The open circuit corrosion potential of the cathode and anode are also measured with respect to a saturated calomel electrode (SCE) after the circuit has been open for two hours to allow the potentials to stabilize. The simulated pore solution, consisting of sodium hydroxide and potassium hydroxide, matches that obtained in a pore solution analysis.(22,23) Epoxy-coated steel is evaluated using specimens in which the coating is breeched by four 3-mm (1/8-inch) diameter holes to simulate defects in the epoxy coating (damaged area = 1.0 percent of total exposed area in the solution). In the rapid macrocell test, bare conventional bars exhibit corrosion initiation within the first 24 hours and conventional bars cast in mortar exhibit corrosion within the first week. The tests last 15 weeks. *The five-week interval is satisfactory to maintain the pH of the solutions above 13.3. Corrosion Rate and Corrosion Loss The corrosion rate of reinforcing steel (measured in the bench-scale tests as well as the rapid microcell tests) indicates how fast reinforcing steel is being oxidized. It may be expressed as a current density, microamps per square centimeter (µA/cm2), which is obtained by measuring the rate of electron flow from anodes to cathodes. Based on Faraday’s law, current density can be converted to another expression for corrosion rate, a rate of loss of metal from the surface of the steel in µm/year.(24) For iron,
As an example of how equation (1) is used to calculate corrosion loss for a bare conventional bar, a voltage drop across the 10-ohm resistor of 0.70 millivolts (mV) = 700 microvolts (µV) represents a total current of 70.0 µA. For a No. 16 (No. 5) bar and a solution depth of 76 mm (the values used in the test), the total surface area in contact with the solution (including the end of the bar) is 4021 mm2 = 40.21 cm2, giving a current density i of 1.74 µA/cm2. Applying equation (1) gives
For zinc, the first coating layer on the MC bars, equation (1) becomes
For reinforced concrete bridge decks, the measurement of the macrocell current is generally not possible because the top and bottom mats of reinforcing steel are usually connected by steel wire ties and bar supports in the concrete slab. In laboratory tests that simulate the corrosion of steel in bridge decks, however, ties and bar supports are not used and the macrocell current can be determined by measuring the voltage drop across a resistor that electrically connects the anode and the cathode through an external circuit.
The measured macrocell current density, and hence the calculated corrosion rate, can be affected significantly by the test methods(25) and the details of the test configuration, such as the anode to cathode area ratio and the size of the resistor connecting the anode and the cathode.(11) Thus, the corrosion rate calculated from the measured macrocell current should be used only to compare the relative performance of corrosion protection systems under same test conditions. Corrosion loss represents cumulative metal loss, expressed in micrometers, and is calculated by numerically integrating the corrosion rate. Test Specimens The specimens in the rapid macrocell test consist of 127 mm (5 inches) long, No. 16 (No. 5) reinforcing bars, either bare or embedded in mortar, as illustrated in figure 4 for epoxy-coated bars. Fabrication of the specimens is described next: (1) Preparation of reinforcing bars: One end of the bar is drilled and tapped 13 mm (0.5 inch) to accommodate a No. 10-24 machine screw. The sharp edges on the bar ends are removed by grinding. Uncoated bars are cleaned with acetone to remove grease and dirt from the surface. For epoxy-coated reinforcing steel, the bars are cleaned with soap and water. The epoxy coating is penetrated by four 3.2 mm (0.125 inch) diameter holes to simulate defects in the coating. The holes are made to a depth of 0.5 mm (0.020 inch) using a 3.2-mm (0.125-inch) diameter fourflute end mill. Two of the holes are placed at the midlength of the bars and the other two are placed about 32 mm (1.25 inch) from the untapped end, which will be submerged in the solution. This submerged end is protected using a plastic cap filled with a repair epoxy. The four holes represent 1 percent damage to the evaluated area of the epoxy-coated bars. Figure 4. Diagram. Rapid macrocell specimens: (a) bare bar and (b) mortar-wrapped specimens with cap to protect the exposed end of epoxy-coated bars. 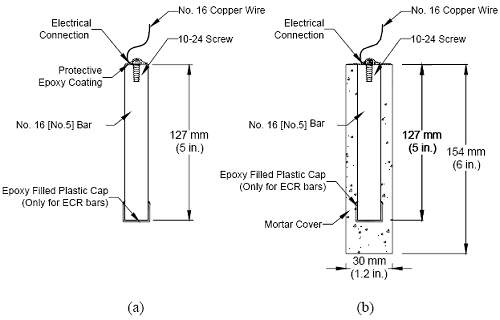
When a mortar-wrapped specimen is used, a prepared bare bar is symmetrically embedded in a 154 mm (6 inch) long mortar cylinder (figure 4b). The cylinder has a 30 mm (1.2 inch) diameter and provides a 7 mm (0.28 inch) mortar cover over the reinforcing bar. Mortar-wrapped bars are cast in a mold consisting of polyvinyl chloride (PVC) pipes and fittings.(19-21) (2) Casting: Mortar is placed in a cylindrical PVC mold in four layers. Each layer is rodded 25 times using a 2-mm (0.08-inch) diameter rod, followed by vibration for 30 seconds on a vibration table with an amplitude of 0.15 mm (0.006 inch) and a frequency of 60 Hz. (3) Curing: Specimens are cured in the molds for one day at room temperature and then removed from the molds and cured in saturated lime water (pH ≈ 12.4) for 13 days to reach a passive condition. After this period, the specimens are surface-dried with compressed air and then vacuum dried for one day. (4) Wiring and coating: For both bare and mortar-wrapped bars, a 16-gage copper electrical wire is attached to the tapped end of each specimen with a 10-24×1/2 (13 mm [0.5 in] long) screw. The electrical connection is then coated with two layers of Herberts O’Brien epoxy for bare bars and two layers of Ceilgard 615™ epoxy by Ceilcote, Inc. for mortar-wrapped specimens. Test Materials (1) Simulated Concrete Pore Solution: Simulated concrete pore solution is used at the cathode. One liter of the solution contains 974.8 grams (g) of distilled water, 18.81 g of potassium hydroxide (KOH), and 17.87 g of sodium hydroxide (NaOH), based on pore solution analysis by Farzammehr et al.(22,23) (2) Simulated Concrete Pore Solution with Sodium Chloride: The solution is used at the anode and is prepared by adding 45.6 or 172.1 g of NaCl to one liter of the simulated concrete pore solution to obtain a 1.6 or 6.04 molal ion concentration solution, equal to a 0.8 or 3.02 molal NaCl solution, respectively. (3) Salt Bridges: A salt bridge provides an ionic path between the cathode and the anode. It consists of a 0.45 m (1.5 ft) long plastic tube filled with a conductive gel. To prepare a salt bridge, 4.5 g of agar, 30 g of potassium chloride (KCl), and 100 g of distilled water are mixed and then heated over a hot plate until the solution starts to thicken. The heated mixture, enough to produce four salt bridges, is poured into plastic tubes using a funnel. The tubes are then placed in boiling water for one hour to firm the gel, keeping the ends of the tubes above the surface of the water. The gel in the salt bridges must be continuous, without interruption by air bubbles. (4) Mortar: The mortar has a water-cement (w/c) ratio of 0.5 and sand-cement ratio of 2.0 by weight, and is made with Type I/II portland cement (ASTM C 150(26)), distilled water, and ASTM C 778(27) graded Ottawa sand. The mix proportions represent the mortar constituent of concrete. The mortar is mixed in accordance with the procedures outlined in ASTM C 305.(28) (5) Mortar Fill: Mortar fill is placed in containers with mortar-wrapped specimens. It consists of the same mixture as used in the test specimens. The fill is cast in a metal baking sheet to a depth of about 25 mm (1 inch). The mortar in the sheet is air-cured at room temperature for 15 days and is broken into 25 to 50 mm (1 to 2 inch) pieces prior to use. Data acquisition A voltmeter with a resolution of 0.001 mV resolution is used to measure corrosion potential of the anode and cathode and the voltage drop across the 10-ohm resistor. The voltage drop tends to fluctuate between –0.003 and 0.003 mV when the corrosion current is close to zero. Only voltage drop readings outside of this region are used to evaluate the corrosion performance of different corrosion protection systems. Values between –0.003 and 0.003 mV are treated as zero. Bench-Scale TestsDuring the past two decades, bench-scale tests, such as the Southern Exposure, ASTM G 109,(29) and cracked beam tests, have been used most often to evaluate the corrosion performance of reinforcing steel.(25,30) Of these tests, the Southern Exposure and cracked beam tests have proven to give useful data in a relatively short period. The ASTM G 109 test gives similar results over a longer period of time, but is perhaps the best choice for corrosion protection systems that contain zinc because of the poor performance of zinc in saturated concrete. All three test methods are used in this study; the results for the first two are reported here. Southern Exposure Test The specimen used in the Southern Exposure, or SE, test(31) consists of a small slab containing two mats of reinforcing steel (figure 5). The concrete is wet cured for three days and then air cured until the test begins at 28 days. The top mat consists of two No. 16 (No. 5) bars; the bottom mat consists of four No. 16 (No. 5) bars. The mats are connected electrically across a 10-ohm resistor, a dam is placed around the edge of the top surface (cast integrally with the specimen in this study), and the sides of the concrete are sealed with epoxy. A 15 percent (6.04 molal ion) sodium chloride solution is placed inside the dam, allowing the chlorides to penetrate into the concrete. The slabs are subjected to a seven day alternate ponding and drying regime, with ponding at 23 ± 2°C (73 ± 3°F) for four days and drying at 100 °F (38 °C) (thus the name Southern Exposure) for three days. Prior to drying, the solution is removed from the upper surface using an industrial vacuum cleaner. The ponding and drying regime is continued for 12 weeks. The specimens are then subjected to continuous ponding for 12 weeks at 23 ± 2 °C (73 ± 3 °F), after which the alternate ponding and drying regime begins again. The two regimes are continued for 96 weeks. Corrosion current and the corresponding corrosion rate are determined by measuring the voltage drop across the resistor. The corrosion potentials of the top and bottom bars are measured.* Corrosion performance is also evaluated using monthly linear polarization resistance readings on selected specimens. The test provides a severe corrosion environment that is generally believed to simulate 15 to 20 years of exposure for marine structures under tropical conditions and 30 to 40 years of exposure for bridges within a 48-week period.(32) Figure 5. Diagram. Southern Exposure test specimen. 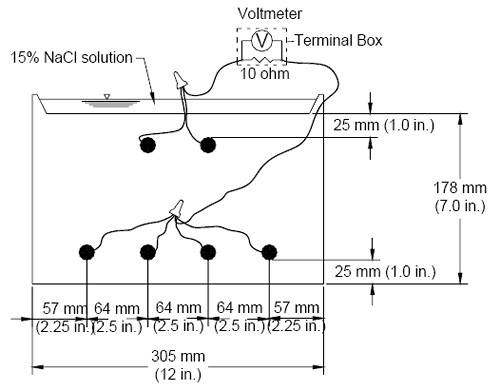 *Corrosion potentials are measured with respect to a saturated calomel electrode (SCE) on ponded specimens and a copper-copper sulfate (CSE) electrode for dry specimens. Potentials with respect to CSE are approximately 0.075 V more negative than those with respect to SCE. Cracked Beam Test The cracked beam specimen (figure 6) is used to model the corrosion of reinforcing steel in which cracks directly expose the steel to deicing chemicals. The specimen is half the width of the SE specimen, with one bar on top and two bars on the bottom. A crack is simulated parallel to and above the top reinforcing bar through the insertion of a 0.3 mm (12 mil) stainless steel shim when the specimen is fabricated. The shim is removed within 12 hours of casting, leaving a direct path for chlorides to the reinforcing steel and simulating the effects of a settlement crack over the bar. An integral dam is used in manner similar to that used for the SE specimen around the upper surface of the specimen. Like the SE specimen, the cracked beam specimen is subjected to cycles of wetting and drying with a 15 percent sodium chloride solution, continuing up to 96 weeks. Figure 6. Diagram. Cracked beam test specimen. 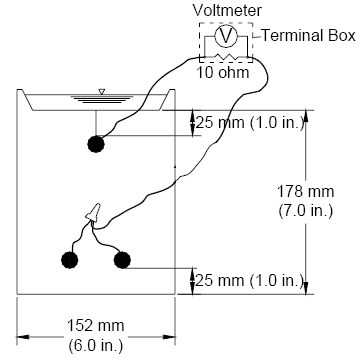 Specimen Fabrication Fabrication of the SE and CB specimens is described next. (1) Preparation of reinforcing bar: Each reinforcing bar is cut to a length of 305 mm (12 inches). Both ends of the bar are drilled and tapped 13 mm (0.5 inch) to accommodate a No. 10-24 machine screw. The sharp edges on the bar ends are removed with a grinder. Uncoated bars are cleaned with acetone to remove grease and dirt from the surface. Epoxy-coated reinforcing bars are cleaned with soap and water. The epoxy coating is then penetrated by four or ten 3.2 mm (0.125 inch) diameter holes to simulate defects in the coating. The holes are made to a depth of 0.4 mm (16 mils) using a 3.2-mm (0.125-inch) diameter four-flute end mill. Two or five holes are placed evenly along the length on each side of the bars. On the multiple coated (MC) bars, specimens are evaluated with both layers penetrated and with only the epoxy penetrated. The epoxy is penetrated without damaging the zinc using a soldering gun set to a temperature of 205 °C (400 °F), which is above the melting temperature of epoxy but below the melting temperature of zinc. The burned epoxy regions are cleaned with acetone. Four holes represent 0.2 percent damage and 10 holes represent 0.52 percent damage to the exposed surface of the epoxy-coated bar. (2) Mold Assembly: The mold is made to cast the specimen in an inverted position. It consists of several pieces of 19 mm (0.75 inch) thick plywood, including 4 sides and a bottom. Inside the mold, a smaller beveled wooden piece is bolted to the bottom to create the integral concrete dam after casting. For the cracked beam molds, a 152 mm (6 inches) long, 0.3 mm (0.012 inch) wide longitudinal slot is made in the center of the beveled wood to accommodate a 0.3 mm (0.012 inch) thick stainless steel shim. The shim projects 25 mm (1 inch) from the slot and just touches the test bar. After demolding, the shim is removed from the concrete to form the crack. All parts of the mold are fastened with angles and clamps. The inside corners are sealed with clay. The bars are supported by 10-24×1 (25 mm [1.0 in] long) screws through 4.8 mm (0.19 inch) diameter holes in two side molds. When epoxy-coated bars are tested, two of the holes in the coating face up and the other two face down. (3) Casting: Concrete is mixed in accordance with the requirements of ASTM C 192(33) for mechanical mixing. The concrete mixture proportions are given in table 5. The concretes have a w/c ratio of 0.45 or 0.35 and a nominal air content of 6 percent. The specimens are cast in two layers. Each layer is vibrated for 30 seconds on a vibrating table with amplitude of 0.15 mm (0.006 inch) and a frequency of 60 Hz. The concrete surface is finished with a wooden float.
(4) Curing: The specimens are cured in the mold for 24 hours at room temperature, except the CB specimens, which usually require earlier demolding (8 to 12 hours) to facilitate the removal of the shim. After being removed from the mold, specimens are cured at room temperature in a plastic bag with water until 72 hours after casting. The specimens are then removed from the bag and air-cured for 25 days. Testing starts 28 days after casting. (5) Wiring and coating: Two days before testing begins, 16-gage copper electrical wire is attached to one end of each bar embedded in the specimens with 10-24×1/2 (13 mm [0.5 inch] long) screw. The other end of the bars is sealed with the same kind of screw. All four sides of the specimens, including the electrical connections, are then coated with two layers of epoxy such as Ceilgard 615™ by Ceilcote, Inc. or ThoRoc SewerGuard HBS™ by ChemRex, Inc. The epoxy is mixed and applied according to manufacturer’s recommendations. Test Materials The properties of the materials are as follows: Type I/II portland cement; coarse aggregate: crushed limestone with maximum size = 19 mm (0.75 inch), bulk specific gravity (SSD) = 2.58, absorption (dry) = 2.27 percent, unit weight = 1536 kg/m3 (95.9 pounds per cubic foot (lb/ft3)); fine aggregate: Kansas River sand with bulk specific gravity (SSD) = 2.62, absorption (dry) =0.78 percent, fineness modulus = 3.18; air-entraining agent: Daravair 1400 by W.R. Grace. A 15 percent NaCl solution (6.04 molal ion concentration) is used to pond the test specimens; 600 ml of the solution is used to pond one SE specimen and 300 ml is used to pond one CB specimen. Data Acquisition The same voltmeter and rules for data conversion described for the rapid macrocell tests are used for the bench-scale tests. Linear Polarization Resistance TestA measure of both microcell and macrocell corrosion can be obtained with the polarization resistance test, which uses a noncorroding counter electrode and a reference electrode to establish a polarization curve by imposing a range of potentials on the metal and measuring the corresponding corrosion currents using a potentiostat. Polarization resistance measurements have been obtained from selected bench-scale specimens throughout the test period. Polarization resistance tests are used in this study to obtain the microcell corrosion rates for bench-scale specimens. In the tests, microcell current readings are taken during a short, slow sweep of bar potential. The sweep typically is from –20 to +20 mV relative to the open circuit potential Eoc. In this range, the current versus voltage curve is roughly linear. The slope of the linear region is proportional to the resistance of the metal. The total corrosion current density then is obtained using the relationship
in which i is the corrosion current density (A/cm2), B is the Stern-Geary constant (typically taken as 26 mV for both reinforcing steel and zinc in concrete), Rp is the slope determined from the polarization curve (kΩ·cm2). The microcell corrosion rate in µm/year (µm/yr) is calculated using Eq. (1) and (2) for iron and zinc, respectively. In this study, the tests are performed using a PC4/750 Potentiostat and DC105 corrosion measurement system from Gamry Instruments. The tests are performed every 4 weeks for bench-scale specimens to obtain the microcell corrosion rates for the top mats with the bottom mats disconnected. In the tests, the top mat of reinforcing steel is used as the working electrode, a saturated calomel electrode immersed in salt solution on the top of the specimen is used as the reference electrode, and a platinum strip immersed in salt solution on the top of the specimen is used as the counter electrode. The data file from a polarization resistance test is analyzed using the data analysis package provided with the DCI 105. This analysis software can read the data file and plot a graph based on the data in the file. When a new graph is created in this package, the user picks a range of voltage in the graph and the software automatically uses a linear fit of the data in the selected range to calculate the polarization resistance. The corrosion current density and corrosion rate can then be determined using the polarization resistance. TEST PROGRAMThe test program, summarized in table 6, compares the corrosion performance of the 11 multiple corrosion protection systems with that of conventional reinforcing steel and conventional epoxy coated reinforcement. As shown in table 6, rapid macrocell and bench-scale tests are used for all systems, but all versions of the tests are not used for every system. In all, the work reported here includes 126 macrocell tests, 93 Southern Exposure tests, and 84 cracked beam tests. Rapid Macrocell Test ProgramAs shown in table 6, the rapid macrocell test with mortar-wrapped specimens is used for all systems, while the macrocell test with bare bars is not used to evaluate the effects of corrosion inhibitors. The macrocell tests for the bare and mortar-wrapped specimens for the multiple coating system include bars with penetrations through both layers, as well as bars in which only the outer layer of epoxy has been penetrated. The coating on each bar in the rapid macrocell tests is penetrated by four 3.2-mm (1/8-inch) diameter holes, representing damage to 1.0 percent the bar area that is exposed to solutions in these tests. Six specimens are used for each system. Bench-Scale Test ProgramSouthern Exposure and cracked beam tests are used to compare the performance of corrosion protection systems cast in concrete. A w/c ratio of 0.45 is used in all cases. In addition, concrete with a w/c ratio of 0.35 is used to evaluate the performance of the corrosion inhibitors (as well as the control specimens) because the performance of concrete containing calcium nitrite has been observed to improve as the w/c ratio decreases.(34) The coatings on bars in the bench-scale tests are penetrated with four or ten 3.2 mm (1/8-inch) holes, representing damage to 0.21 and 0.52 percent of the bar surface, respectively.
Bench-Scale Test ProgramSouthern Exposure and cracked beam tests are used to compare the performance of corrosion protection systems cast in concrete. A w/c ratio of 0.45 is used in all cases. In addition, concrete with a w/c ratio of 0.35 is used to evaluate the performance of the corrosion inhibitors (as well as the control specimens) because the performance of concrete containing calcium nitrite has been observed to improve as the w/c ratio decreases.(34) The coatings on bars in the bench-scale tests are penetrated with four or ten 3.2 mm (1/8-inch) holes, representing damage to 0.21 and 0.52 percent of the bar surface, respectively. Following the tests, specimens are photographed to record cracking and corrosion products visible on the exterior of concrete or mortar and corrosion products on reinforcing steel and surrounding cementitious materials following the removal of concrete or mortar. Linear Polarization Resistance Test ProgramLinear polarization resistance measurements are performed on a single Southern Exposure and cracked beam specimen for each configuration and corrosion protection system in the study (table 6). The results are used in conjunction with the readings obtain from the macrocell and bench-scale tests to evaluate the performance of the corrosion protection systems.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||