U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| TECHBRIEF |
| This techbrief is an archived publication and may contain dated technical, contact, and link information |
| Publication Number: FHWA-HRT-15-056 Date: June 2017 |
Publication Number: FHWA-HRT-15-056 Date: June 2017 |
PDF Version (300 KB)
PDF files can be viewed with the Acrobat® Reader®
| FHWA Publication No.: FHWA-HRT-15-056 FHWA Contact: Jack Youtcheff, HRDI-10, (202) 493-3090, jack.youtcheff@dot.gov |
Excess water in asphalt binders and pavement mixes can have a deleterious effect on pavement performance.(1) The addition of water to binders through the use of warm and cold mix technologies highlights the need for accurately measuring water in asphalt. Further, increased water content of biologically derived binders and oils may be responsible for compatibility issues with petroleum-based products. Several analytical techniques have been developed for quantifying water in bituminous material, including the use of Fourier transform infrared spectroscopy (FTIR), gas chromatography (GC), nuclear magnetic resonance (NMR), and others that vary in accuracy, cost, sensitivity, specificity, and ease of operation.
Application of these methods to asphalt has proven difficult for a variety of reasons. Subsequent to evaluation of GC, FTIR, and NMR techniques, the Karl Fischer (KF) method, which is the “gold standard” for measuring the water content in lubricating oils, was considered. KF titration was not considered appropriate for measuring the water in asphalt because of interference reactions with mercaptans, sulfides, and ketones. With innovations in KF reaction solutions such as Hydranal® and Aquastar®, there was new hope that KF titration could be applied to bituminous material. In preliminary studies, samples of asphalt were sent to a commercial laboratory for KF analysis. Inconsistent results suggested that atmospheric water may have contaminated the samples. New laboratory techniques—including storing samples, solvents, and glassware in a dry atmosphere and performing all sample preparation in a dry environment—were employed, and these techniques subsequently improved repeatability to ± 20parts per million (ppm)/sample.
A Mettler Toledo® coulometric C20 KF titrator and Hydranal®-Coulomat reagents (Sigma-Aldrich™ pt# 34840-50ML-R and 34868-500ML-R) were used for all titrations. Coulomat reagents are designed to minimize interfering reactions that prevent accurate titration of samples containing mercaptan and ketone functional groups. In addition, the fast reaction rate and quick titration prevent the relatively slow interference reactions from causing appreciable error. Coulometric KF titrators use an electric current to generate iodine (I2) from iodide (2I-) in solution, which reacts rapidly with water as described by figure 1. A sudden voltage drop indicates free iodine in solution and the titration end point. Using Faraday’s law, one can determine that the current required to reach the end point is proportional to the iodine generated. This method and procedure is suitable for solutions with low moisture content (10 to 50,000 ppm).
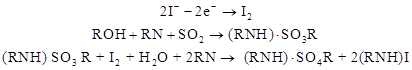
Figure 1. Equation. Generic chemical equation for KF titration.
All sample preparation was performed in a glove box with dry nitrogen atmosphere. A plastic glove bag (Sigma-Aldrich™ pt# Z530212 or similar) may be used if a glove box is not available. Addition of Drierite® or activated silica further dried the air inside the glove box. All glassware was heated at 140 °C for at least 1 h, and while the glassware was still hot, it was placed inside the glove box with constant nitrogen purging and allowed to cool. One g of asphalt and 4 g of anhydrous toluene (stored over activated molecular sieves) were added to a 10 mL glass vial and capped with a Teflon® crimp lid to generate a 20-percent wt/wt solution. After the asphalt dissolved, at least 3 g of solution were injected into the KF reaction vessel with a dry disposable syringe (Restek Norm-Ject® pt# 22769 or similar) and hypodermic needle and allowed to react. The quantity of sample injected was measured using an analytical balance, and the parts per million water in solution result was used to calculate the parts per million water in asphalt. During this process, only very dry nitrogen contacted the toluene, asphalt solution, and cooled glassware, minimizing the chances of contamination by atmospheric moisture.
Debate over the applicability and effectiveness of various emulsion recovery procedures is evidenced by industry’s struggle to develop a standard method.(2,3) In this study, three methods were employed to recover asphalt emulsion residue in the laboratory, including a novel approach developed at Western Research Institute. The first, American Association of State Highway and Transportation Officials PP72-11 method B (Standard Practice for Recovering Residue from Emulsified Asphalt Using Low-Temperature Evaporative Techniques), involves spreading a -381-μm-thick emulsion layer with a fixed thickness spreader bar onto a rubber mat and drying the mat in a forced draft oven at 60 °C for 6 h.(4) The second method uses the equivalent mass of emulsion to achieve a 300-μm-thick recovered emulsion residue on a Universal Simple Aging Test (USAT) aluminum plate and drying it in a vacuum oven at 60° C for 2 h.(3) A TechBrief on USAT is also available.(5) The third method also uses equivalent mass of emulsion to achieve a 300-μm-thick recovered emulsion residue on a USAT plate and a forced draft oven at 60 °C for 6 h.(6) The 300-μm residue thickness was chosen so that laboratory aging of the sample could occur without transfer to a separate container for standard (R28) pressuring aging vessel (PAV) aging. In a separate study, it was demonstrated that aging a 300-μm asphalt film on a USAT plate under standard PAV conditions for 8 h resulted in equivalent oxidation to R28, which requires a 3.2-mm-thick film and conditioning for 20 h.(6) Emulsions used in this study were named according to ASTM D2397, with the addition of LM or P denoting latex modified or polymer modified.(7)
Several samples of 300-µm-thick recovered emulsion residue and neat binders were treated in a 100-percent humidity atmosphere at 60 °C for 2 weeks in a forced draft oven in an attempt to accurately determine the amount of remaining moisture in the residue after recovery. The samples were then analyzed by KF titration to determine the equilibrium water content of each sample.
Before the application of the KF method to asphalt, the effectiveness of emulsion recovery was evaluated by drying a sample to constant mass and quantifying residual water in the residue by calculating mass loss. This method of water quantification in emulsion residue was evaluated by comparing KF results with mass lost after drying at 135 °C.
Results from KF titration of Strategic Highway Research Program (SHRP) core binders are listed in figure 2 and are given in ppm water or µg water/g solution. The binders were from sealed cans that had been heated, sampled, and capped with argon several times over the past 15 years. It was still unclear whether the measured KF values were the result of interfering reactions between the Coulomat reagents and functional groups within asphalt or water in the binder. To address this issue, a minimum-boiling azeotrope (or positive azeotrope) of water and toluene was formed by dissolving approximately 5 g of asphalt in approximately 50 mL of toluene and evaporating the solvent on a rotary evaporator with vacuum and nitrogen purge. When very low concentrations (< 1 percent) are present from the outset, the distillate will beenriched in water compared with the remaining distilland (asphalt), which will be virtually free of water. The KF results for these azeotropic dried samples showed, on average, nearly 70 ppm water remaining. While it is likely that some portion of the measured water in asphalt is the result of interference reactions, the reduction of water in the azeotroped samples indicates that at least a portion of the very small KF response in the neat binders is the result of moisture. It is estimated that 75 to 175 ppm water exists in an untreated binder because this is the difference between the neat binder and the azeotrope dried binders. AAD‑1 and AAC‑1 PAV aged 3.2-mm films at 100 °C for 100 h were titrated based on the theory that an increase in ketones may cause a change in the titration end point. Aged AAD‑1 azeotroped showed nearly double the amount of water compared with unaged AAD‑1, indicating that interference reactions are likely occurring during KF titration. From the samples tested to date, a maximum of 170 ppm water false positive is the result of interference, and this number is more likely much lower in the binder that has not been subjected to oxidative aging.
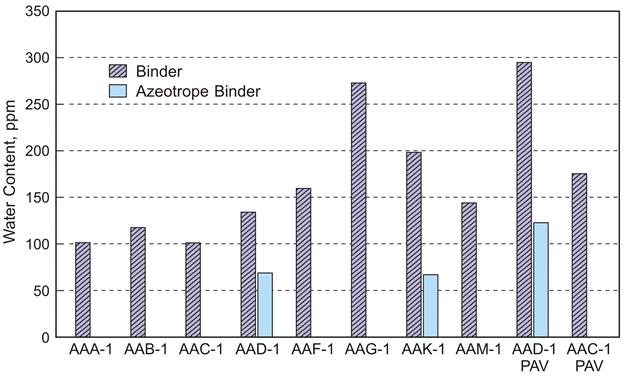
Source: WRI.
Figure 2. Chart. SHRP binders water content by KF titration.
Figure 3 shows the amount of the water in each emulsion residue with the respective recovery method. No clear trends in water content are apparent with a specific emulsion or recovery method. It is probable that differences in water content between residues and recovery method are primarily due to variations in sample preparation on the rubber mat or USAT plate. Data collected by Reinke et al. suggesting that less than 1-percent water (wt/wt) is the threshold level where the viscosity of a recovered emulsion matches that of the base binder used to create the emulsion, which indicates that all three methods are satisfactory for emulsion recovery.(3)
Reinke’s method for determining water content in residues involves heating the residue at 135° C until constant mass is obtained and then calculating water content from mass lost during 135° C drying. KF titration values on recovered residues before drying at 135° C were lower than water content by measured weight loss for the four residues sampled. For one CSS-1H residue, the discrepancy was as much as 9,000 ppm. Recovered residues before and after drying at 135 °C were analyzed by mass spectrometry with the hypothesis that some volatile additive other than water was evaporated while at elevated temperature. After mass spectrometry analysis, it was determined that a significant amount of 2,2,oxybis ethanol (90-percent match in the NIST98 library) was evaporated from the CSS-1H residue with the 9,000 ppm water content discrepancy. Some of this compound was also present in other residues with water content discrepancies less than 3,000 ppm but was completely evaporated by exposure to 135 °C temperatures. These data are a possible explanation for the conflicting results of the two methods of water quantification in residues and support the assertion that the KF method is more accurate for a wider variety of asphalt emulsions. Time did not permit a more exhaustive study looking for volatiles in all eight emulsion residues. The reason 2,2,oxybis ethanol was added to these emulsions is unknown, but this compound may be an antifreeze agent or cutback.
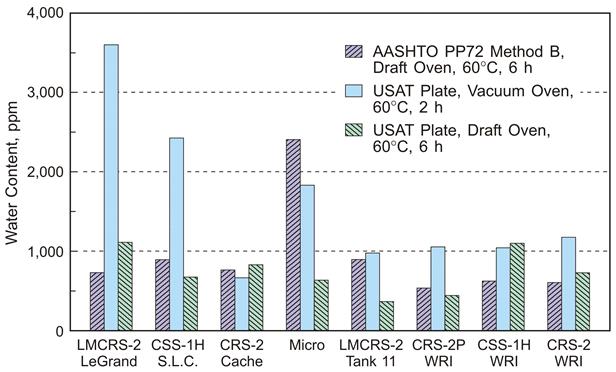
Source: WRI.
Figure 3. Chart. Water content of eight emulsion residues after three recovery methods.
Because the less than 1-percent water content for effectively recovering emulsion residue may be inaccurate because of non-water volatile loss in the 135 °C oven, more effort was made to understand the maximum quantity of water remaining in emulsion residues before rheological testing. Several USAT plates were prepared with 300-μm-thick recovered emulsion residue or neat binder and treated in a 100-percent humidity atmosphere at 60 °C for 2 weeks. The samples were then analyzed by KF titration to determine the equilibrium water content. The results are presented in figure 4. Assuming that the samples had sufficient time in the humidity chamber, the equilibrium water content was not significantly elevated in any of the samples treated in the humidity chamber. Two of the USAT plate vacuum oven recovered residues (60 °C for 2 h) had more water than the equilibrium water content of the same residues. Perhaps 3 h in the vacuum oven at 60 °C would ensure sufficient removal of water for all emulsion residues.
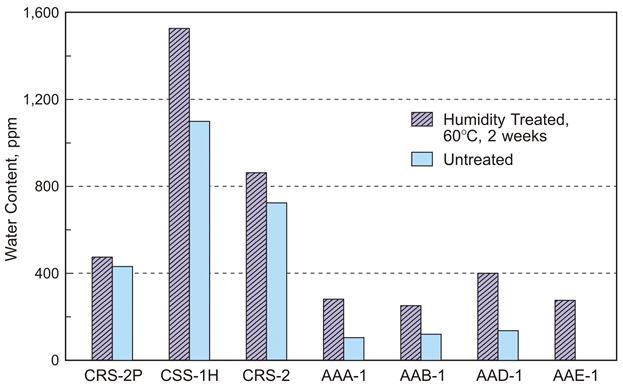
Source: WRI.
Figure 4. Chart. Untreated asphalt and recovered asphalt emulsion residue water content
after treatment for 2 weeks in a 60 °C oven with 100-percent humidity in a USAT plate.
Excess water in asphalt binders and pavement mixes can have a deleterious effect on pavement performance.(1) The addition of water to binders through the use of warm and cold mix technologies highlights the need for accurately measuring water in asphalt. Furthermore, increased water content of biologically derived binders and oils may be responsible for compatibility issues with petroleum-based products. A KF titration method for quantifying water in asphalt and asphalt emulsions has been developed that is accurate, quick, and highly sensitive. The method's detection limit is approximately100 ppm. SHRP binders were tested to assess the effectiveness of the method, and neat binder water content varied from 100 to 275 ppm water. The method was applied in the evaluation of three emulsion recovery procedures and indicated that each procedure removed sufficient water for rheological measurement of the residues. More testing with the USAT plate vacuum oven method or more time in the oven may be necessary to ensure ruggedness for all asphalt emulsions. Binder films and emulsion residue films (300 μm thick) that were treated in a 60 °C, 100-percent humidity environment showed a maximum of 1,500 ppm water and generally contained approximately 200 ppm more water than non-humidity-treated films. The method of quantifying water in emulsion residues by heating at 135°C until constant mass is obtained and calculating water content by mass loss was evaluated using KF titration. It was determined that some residues may contain volatile material other than water that is evaporated at 135 °C, yielding artificially elevated and inaccurate water content measurements by the mass loss method.
|
Researchers—This TechBrief was prepared by WRI and is the result of several years of research at WRI with the support of FHWA. Distribution—This TechBrief is being distributed according to a standard distribution. Direct distribution is being made to the Divisions and Resource Center. Availability—The report may be obtained from the FHWA Product Distribution Center by e-mail to report.center@fhwa.dot.gov, fax to (301) 577-1421, phone to (301) 577-0818, or online at http://www.tfhrc.gov/safety. Key Words— Asphalt water content, Karl Fischer analysis, USAT, emulsion recovery. Notice—This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The U.S. Government assumes no liability for the use of the information contained in this document. The U.S. Government does not endorse products or manufacturers. Trademarks or manufacturers’ names appear in this report only because they are considered essential to the objective of the document. Quality Assurance Statement—The Federal Highway Administration (FHWA) provides high-quality information to serve the Government, industry, and public in a manner that promotes public understanding. Standards and policies are used to ensure and maximize the quality, objectivity, utility, and integrity of its information. FHWA periodically reviews quality issues and adjusts its programs and processes to ensure continuous quality improvement. |