U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-RD-97-148 |
| [ Asphalt Concrete ] | [ Granular Base ] | [ Embankment or Fill ] |
ORIGIN
Nonferrous slags are produced during the recovery and processing of nonferrous metal from natural ores. The slags are molten by-products of high temperature processes that are primarily used to separate the metal and nonmetal constituents contained in the bulk ore. When cooled, the molten slag converts to a rocklike or granular material.
The processing of most ores involves a series of standardized steps. After mining, the bulk ore is processed to remove any gangue (excess waste rock and minerals). This processing typically consists of pulverizing the ore to a relatively fine state, followed by some form of gravity separation of the metals from the gangue (using a series of devices including cyclone separators, inclined vibratory tables, and flotation tanks). The refined ore is processed thermally to separate the metal and nonmetal constituents, then further reduced to the free metal. Since most of these metals are unsuitable for use in a pure state, they are subsequently combined with other elements and compounds to form alloys having the desired properties.
In preparation for metal ion reduction (designed to separate the metal from the nonmetal constituents), some nonoxide minerals are often converted to oxides by heating at air temperatures below their melting point ("roasting"). Sulfide minerals, when present (in copper and nickel ore), are converted to oxides in this process. The reduction of the metal ion to the free metal is normally accomplished in a process referred to as smelting. In this process, a reducing agent, such as coke (impure carbon), along with carbon monoxide and hydrogen, is combined with the roasted product and melted in a siliceous flux. The metal is subsequently gravimetrically separated from the composite flux, leaving the residual slag.
The following nonferrous slags are included in these guidelines: copper, nickel, phosphorus, lead, lead-zinc, and zinc. Approximately 3.6 million metric tons (4 million tons) each of copper and phosphorus slag are produced each year in the United States, while the annual production of nickel, lead and zinc slags is estimated to be in the range of 0.45 to 0.9 million metric tons (0.5 to 1.0 million tons).(1)
Copper and Nickel Slags
Copper and nickel slags are produced by: (1) roasting, in which sulfur in the ore is eliminated as sulfur dioxide (SO2); (2) smelting, in which the roasted product is melted in a siliceous flux and the metal is reduced; and (3) converting, where the melt is desulfurized with lime flux, iron ore, or a basic slag and then oxygen lanced to remove other impurities.
Copper slag that is derived by smelting of copper concentrates in a reverberatory furnace is referred to as reverberatory copper slag.(1)
Phosphorus Slag
Phosphorus slag is a by-product of the elemental phosphorus refining process. The elemental phosphorus is separated from the phosphate-bearing rock in an electric arc furnace, with silica and carbon added as flux materials to remove impurities during the slagging process. Iron, which is added to the furnace charge, combines with phosphorus to form ferrophosphorus which can be tapped off. The slag, which remains after removal of elemental phosphorus and/or ferrophosphorus, is also tapped off.
Lead, Lead-Zinc and Zinc Slags
Lead, lead-zinc, and zinc slags are produced during pyrometallurgical treatment of the sulfide ores. The process includes three operations similar to copper and nickel slag production: (1) roasting, (2) smelting, and (3) converting. Lead and zinc are often related as coproducts in both source and metallurgical treatments, and the various combinations of slags, which include lead, lead-zinc, and zinc, are similarly produced.
Figure 11-1 presents a general schematic depicting the slag production process for copper, nickel, and lead-zinc slags. Figure 11-2 presents a similar diagram for phosphorus slag production.
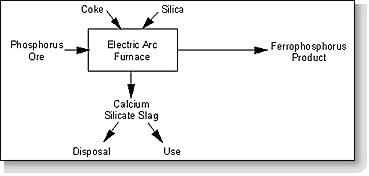
Figure 11-1. General process diagram for copper, nickel and lead-zinc slag production.
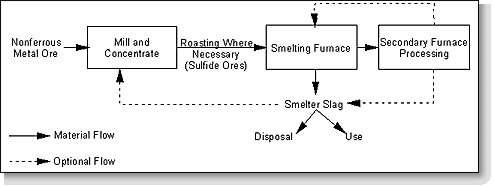
Figure 11-2. General process diagram for phosphorus slag production.
CURRENT MANAGEMENT OPTIONS
Recycling
Copper and Nickel Slags
Copper and nickel slags have been used as granular base and embankment materials, aggregate substitutes in hot mix asphalt, mine backfill materials, railway ballast materials, grit blast abrasives, roofing granule material, and in the manufacture of blended cements (granulated copper and nickel slags).(2,3)
Phosphorus Slag
Phosphorus slag has been used as an aggregate substitute in hot mix asphalt, as a lightweight masonry aggregate, and as cement kiln feed. Granulated phosphorus slag has also been used experimentally in the production of a blended cement product.(4)
Lead, Lead-Zinc, and Zinc Slags
Some zinc slags have reportedly been used in the manufacture of ceramic tiles and as an aggregate substitute in hot mix asphalt.(5,6)
Disposal
Nonferrous slags are produced in a few locations, often remote from potential markets. As a result, nonferrous slags are not well utilized and most of the nonferrous slag produced is disposed of in slag dumps or stockpiles.
MARKET SOURCES
Nonferrous slags, when they are used, are marketed directly by the producers. Slag generation is highly dependent on specific processes and sources; consequently, slag properties can vary between plants and different ore sources, and must be investigated on a case-by-case basis.
Most of the molten nonferrous slag that is produced is dumped into a pit and simply allowed to air cool, solidifying under ambient conditions. A small proportion is granulated, using rapid water and air quenching that results in the production of a vitrified fritlike product. Phosphorus slag is sometimes expanded (solidified with controlled quantities of water, air, or steam, which accelerate the process and increase the cellular nature of the slag), producing a lightweight product.
The cooling rate has a strong influence on the mineralogy and, consequently, the physical and cementitious properties of the nonferrous slags. Granulated slags, which are produced by rapid quenching of the molten slag, are more vitreous and more reactive than similar air-cooled slags. Granulated slags solidify to relatively small, uniform particles whereas air-cooled slags solidify in a large mass.
HIGHWAY USES AND PROCESSING REQUIREMENTS
Because they are produced in remote geographic locations, nonferrous slags are not commonly used in highway construction applications. Nonetheless, there have been reported uses of nonferrous slag as an aggregate substitute in hot mix asphalt and granular base applications.
Asphalt Concrete Aggregate
Phosphorus, copper, and nickel slags have been used as aggregate substitutes in hot mix paving. Air-cooled slags can be used as coarse or fine aggregate, while granulated slags can be used as fine aggregate.(1)
Granular Base, Embankment, and Fill
There has been limited use of copper, nickel, and phosphorus nonferrous slags as a granular base material. (See references 7,8,9,10 and 11.)
Nonferrous slags have the potential for use as an aggregate in embankments, although there is little documentation of use in this application. (See references 9,10,11,12,13 and 14.)
Processing of nonferrous air-cooled slags for use as aggregate involves conventional crushing and screening to meet the specified gradation requirements. Granulated slag particles are generally of fine aggregate size and may require blending with other suitable material to satisfy specified gradation requirements. Granulated copper and nickel slags can be expected to exhibit some cementitious properties similar to granulated phosphorus slags; however, there is no documented use of these slags in this (cementing) capacity.
MATERIAL PROPERTIES
Physical Properties
Table 11-1 lists some typical physical properties for nonferrous slags. Because they have similar properties, lead, lead-zinc, and zinc slags are grouped together.
Table 11- 1. Typical physical properties of nonferrous slags.
| Property | Nickel Slag | Copper Slag | Phosphorus Slag | Lead, Lead-Zinc, and Zinc Slags |
| Appearance | Reddish brown to brown-black, massive, angular, amorphous texture |
Black, glassy, more vesicular when granulated |
Black to dark gray, air-cooled is flat and elongated but granulated is uniform, angular | Black to red, glassy, sharp angular (cubical) particles |
| Unit weight, (kg/m3) |
3500(13) | 2800 to 3800(15) | Air-cooled: 1360 to 1440 Expanded: 880 to 100(16) |
< 2500(17) to 3600(15) |
| Absorption (%) | 0.37(13) | 0.13(10) | 1.0 to 1.5(18) | 5.0(16) |
Nickel Slag
Air-cooled nickel slag is brownish-black in color. It crushes to angular particles but has a smooth, glassy texture. The specific gravity of air-cooled nickel slag may be as high as 3.5(15), while the absorption is quite low (0.37 percent).(3) The unit weight of nickel slag is somewhat higher than that of conventional aggregate.
Granulated nickel slag is essentially an angular, black, glassy slag "sand" with most particles in the size range of minus 2 mm (No. 10 sieve) to plus 0.150 mm (No. 100 sieve).(15) It is more porous, with lower specific gravity and higher absorption, than air-cooled nickel slag.
Copper Slag
Air-cooled copper slag has a black color and glassy appearance. As a general rule, the specific gravity will vary with iron content, from a low of 2.8 to as high as 3.8.(15) The unit weight of copper slag is somewhat higher than that of conventional aggregate. The absorption of the material is typically very low (0.13 percent).(17)
Granulated copper slag is more porous and therefore has lower specific gravity and higher absorption than air-cooled copper slag. The granulated copper slag is made up of regularly shaped, angular particles, mostly between 4.75 mm (3/4 in) and 0.075 mm (No. 200 sieve) in size.
Phosphorus Slag
Air-cooled phosphorus slag tends to be black to dark gray, vitreous (glassy), and of irregular shape. Individual particles are generally flat and elongated, with sharp fracture faces similar to broken glass. The crushed material has a unit weight of 1360 to 1440 kg/m3 (85 to 90 lb/ft3)(1),13 which is less than that of conventional aggregate, with absorption values of about 1.0 to 1.5 percent.(10) Expanded phosphorus slag has a unit weight of 880 to 1000 kg/m3 (55 to 62 lb/ft3)(13) and has a higher absorption than air-cooled slag due to its more vesicular nature.
Granulated phosphorus slag is made up of regularly shaped, angular particles, mostly between 4.75 mm (1/4 inch) and 0.075 mm (No. 200 sieve) in size. It is more porous than air-cooled slag and consequently has lower specific gravity and higher absorption.
Lead, Lead-Zinc, and Zinc Slags
Slags of this group are often black to red in color and glassy. They have sharp, angular particles that are cubical in shape. The unit weights of lead, lead-zinc, and zinc slags are somewhat higher than conventional aggregate materials.
Granulated lead, lead-zinc, and zinc slags tend to be porous, with up to 5 percent absorption.(16) The specific gravity can vary from less than 2.5(16) to as high as 3.6.(15) These slags are made up of regularly shaped, angular particles, mostly between 4.75 mm (3/4 in) and 0.075 mm (No. 200 sieve) in size.
Chemical Properties Copper, lead, lead-zinc, and zinc slags are essentially ferrous silicates, while phosphorus slag and nickel slag are primarily calcium/magnesium silicates. Table 11-2 list typical chemical compositions of these slags.
Table 11-2. Typical chemical compositions of nonferrous slag, %.(14)
| Element | Reverberatory Copper Slag | Nickel Slag | Phosphorus Slag | Lead Slag | Lead-Zinc Slag |
| SiO2 | 36.6 | 29.0 | 41.3 | 35.0 | 17.6 |
| Al2O3 | 8.1 | trace | 8.8 | – | 6.1 |
| Fe2O3 | – | 53.06 | – | – | – |
| CaO | 2.0 | 3.96 | 44.1 | 22.2 | 19.5 |
| MgO | – | 1.56 | – | – | 1.3 |
| FeO | 35.3 | – | – | 28.7 | – |
| K2O | – | – | 1.2 | – | – |
| F | – | – | 2.8 | – | – |
| MnO | – | trace | – | – | 2.0–3.0 |
| P2O5 | – | – | 1.3 | – | – |
| Cu | 0.37 | – | – | – | – |
| BaO | – | – | – | – | 2.0 |
| SO3 | – | 0.36 | – | – | – |
| Free CaO | – | – | – | – | – |
| S | 0.7 | – | – | 1.1 | 2.8 |
| PbO | – | – | – | – | 0.8 |
During slag production, the sudden cooling that results in the vitrification of nonferrous slags (typically in the granulating process) prevents the molecules from being locked up in crystals. In the presence of an activator (such as calcium hydroxide from hydrating Portland cement) vitrified nonferrous slags react with water to form stable, cementitious, hydrated calcium silicates. The reactivity depends on the fineness to which the slag is ground (reactivity increases with fineness)(4) and the chemical composition of the slag and its glass content. These vitrified slags can be of such composition that when ground to proper fineness, they may also react directly with water to form hydration products that provide the slag with cementitious properties. A high iron content (essentially ferrous silicate slags) in these slags appears to limit hydraulicity and makes grinding difficult.
There is some evidence that nickel slag can be involved in the corrosion of iron and steel in the presence of moisture (probably galvanic corrosion). In Canada, where nickel slag is used in fill applications, it is common practice to provide a layer (typically 150 mm (6 in) thick) of natural aggregate between ferrous materials and the slag.(15)
Hydratable oxides may also be present in some nonferrous slags from some sources, which could potentially contribute to volumetric instability.
Depending on the ore and metallurgical process, nonferrous slags produced from sulfide ores can contain leachable elemental sulfur and heavy metals, which should be investigated prior to use. Sulfurous leachate is primarily of aesthetic concern, resulting in sulfur odor and possible discoloration of water in poor drainage conditions. In addition, phosphate rocks can contain between 30 and 200 ppm uranium. Most of this uranium is incorporated in the phosphorus slag and results in the release of some radiation (in the form of radon gas), although Tennessee Valley Authority tests have shown that the level of radiation does not appear to present a significant hazard.()
Mechanical Properties
Table 11-3 presents typical mechanical properties for nonferrous slags. Processed air-cooled and granulated copper, nickel, and phosphorus slags have a number of favorable mechanical properties for aggregate use, including excellent soundness characteristics, good abrasion resistance, and good stability (high friction angle due to sharp, angular shape). However, nonferrous slags tend to be vitreous, or "glassy," which adversely affects their frictional properties (skid resistance), a potential problem if used in pavement surfaces.
Table 11-3. Typical mechanical properties of nonferrous slags.
| Test | Nickel Slag | Copper Slag | Phosphorus Slag | Lead, Lead-Zinc, and Zinc Slags |
| Los Angeles Abrasion Loss, % | 22.1(17) | 24.1(17) | < 30(10) | No data |
| Sodium Sulfate Soundness Loss, % | 0.40(17) | 0.90(17) | < 1(10) | No data |
| Angle of Internal Friction | ~ 40° | 40 - 53(11) | No data | No data |
| Hardness (measured by Moh's scale of mineral hardness) | 6 - 7(17) | 6 - 7(17) | No data | No data |
REFERENCES
Collins, R. J. and S. K. Ciesielski. Recycling and Use of Waste Materials and Byproducts in Highway Construction, National Cooperative Highway Research Program Synthesis of Highway Practice No. 199, Transportation Research Board, Washington, DC, 1994.
Emery, J. J., "Dominican Republic Mega Project Uses Hi-Tech Hot Mix," Ontario Hot Mix Producers Association, (OHMPA), Asphaltopics, Volume 8, Issue 2, July 1995.
Ontario Ministry of Natural Resources. Mineral Aggregate Conservation Reuse and Recycling. Report prepared by John Emery Geotechnical Engineering Limited (JEGEL) for Aggregate and Petroleum Resources Section, Ontario Ministry of Natural Resources, Ontario, 1992.
Tennessee Department of Transportation. Test Reports on Samples of Coarse and Fine Aggregates, Provided to JEGEL, July, 1995.
Hughes, M.L. and T.A. Halliburton, "Use of Zinc Smelter Waste as Highway Construction Material," Highway Research Record No. 430, 1973, pp. 16-25.
Gutt, W., P. J. Nixon, M. A. Smith, W. H. Harrison and A. D. Russell. A Survey of the Locations, Disposal and Prospective Uses of the Major Industrial Byproducts and Waste Materials. CP 19/74, Building Research Establishment, Watford, U.K., 1974.
Miller, R. H. and R. J. Collins. Waste Materials as Potential Replacements for Highway Aggregates. National Cooperative Highway Research Program Report 166, Transportation Research Board, Washington, DC, 1976.
Wrong, G. A., Experiences with Inco Slag for Highway Construction Purposes. Ontario Department of Highways Report, January, 1960.
Queneau, P. B., L. D. May, and D. E. Cregar, "Application of Slag Technology to Recycling of Solid Wastes." Incineration Conference, Knoxville, Tennessee, May, 1991.
Feasby, D.G. Mineral Wastes as Railroad Ballast. Canada Centre for Mineral and Energy Technology, National Mineral Research Program, Mineral Sciences Laboratories Report MRP/MSL 75-76 (OP), Ottawa, Canada, 1975.
Das, B. M., A. J. Tarquin, and A. Q. Jones. "Geotechnical Properties of Copper Slag," Transportation Research Record No. 941, National Research Board, Washington, DC, 1993.
Gallup, G. H., White Pine Copper Company’s Reverberatory Furnace Slag for Highway Aggregates. Michigan Department of State Highways and Transportation, Testing and Research Division, October, 1974, p. 25.
Emery, J. J., "Slag Utilization in Pavement Construction," Extending Aggregate Resources, ASTM Special Technical Publication 774, American Society for Testing and Materials, 1982, pp. 95-118.
OECD. Use of Waste Materials and By-Products in Road Construction. Organization for Economic Co-operation and Development, Paris, 1977.
JEGEL. Manitoba Slags, Deposits, Characterization, Modifications, Potential Utilization. Report prepared by John Emery Geotechnical Engineering Limited, Toronto, Ontario, 1986.
Mantell, C. L. Solid Wastes: Origin, Collection, Processing and Disposal. John Wiley & Sons, New York, 1975.
Hughes, M.L. and T.A. Haliburton, "Use of Zinc Smelter Waste as Highway Construction Material," Highway Research Record No.430, 1973, pp.16-25.
Petty, F., Tennessee Department of Transportation, personal communication, July 1995.
Mag, A. and J. J. Boyle. Assessment of Ra226 and Toxic Element Distribution at Tennessee Valley Authority Phosphate Slag Stockpiles, Muscle Shoals, AL. Report of Investigations/1990 RI 9288, United States Bureau of Mines, Washington, DC, 1990.
| [ Asphalt Concrete ] | [ Granular Base ] | [ Embankment or Fill ] |