U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-04-150 Date: July 2006 |
Previous | Table of Contents | Next
The concrete petrographer is often called upon to ascertain the presence or absence of particulate materials other than portland cement in a specimen of HCC. These materials include all of the finely comminuted solid substances that may be added to HCC to improve its properties or reduce its cost. These substances are of relatively small grain size, usually at least as small as that of portland cement. Included are pulverized limestone (Kleiger and Hooton, 1988), hydrated lime, natural pozzolans, fly ash, slag, and silica fume (see ACI 232.1R, 232.2R, 233R, and 234R; Cain, 1994; TRB, 1990).
In general, when these materials have been used in a concrete mixture as prescribed, the resulting concrete has higher ultimate strength and is less permeable than a similar concrete that does not include these materials. Historically, some of these substances were considered to be adulterants and their use was considered to be an effort to dilute the cement and produce a less costly, less energy-intensive product (e.g., ground limestone). However, materials such as ground slag or pozzolans improve key HCC properties that directly impact durability. A potential drawback to their use when the mixture is not proportioned properly is delayed strength gain (except with silica fume and other high-fineness pozzolans). In cold weather, this delay can be sufficient to mandate against their use.
These materials should not be considered as adulterants when they have been specified for use. The particulate materials that are rich in amorphous silicates (slag, fly ash, silica fume, and natural pozzolan) are hydraulic (slag) or pozzolanic and will combine with the calcium hydroxide of HCC to form silicate hydrates that are cementitious and indistinguishable from the hydrated products of portland cement. The reaction of these materials cause the sequestration of deleterious alkalis in nonswelling silicate hydrates (see appendix D) that are widely disseminated throughout the paste. Thus, these pozzolanic materials prevent the deterioration that might be caused by the AARs provided that they are used in sufficient amounts.
The fine grain size; slow hydration; and, in some cases, particular particle shape add desirable properties to HCC. The heat of hydration is generated more slowly and, therefore, the temperature of the HCC does not rise so high. One of the most important desirable properties conferred by these materials is the decreased permeability of the resulting HCC. The decreased permeability is not only desirable in and of itself, it also decreases the movement of solutions and the diffusion of ions and thus any chemical activity, such as that of alkali reactivity or the activity of invasive deleterious substances such as chlorides. Pozzolanic materials are reported to be effective in increasing the acid resistance of concrete (Cain, 1994). Natural pozzolans and fly ash should comply with the requirements of ASTM C 618, slag with ASTM C 989, and silica fume with ASTM C 1240. Pozzolans and slag may also be used as components in hydraulic cements covered by ASTM C 595 and ASTM C 1157.
The appearance of a mature concrete in which these materials have been used is generally that of concrete with a low w/cm (see chapter 9). Pulverized limestone, cement kiln dust, or hydrated lime may be suspected in HCC that appears to have the texture of an HCC with a low w/cm and numerous minute particles of high birefringence when viewed in thin sections with crossed 182 polarized light using a petrographic microscope. It is impossible to be certain with mature concrete and without any magnification if fly ash, slag, or silica fume is present or if the paste characteristics are solely a result of a low w/cm.
Detecting the presence of pozzolans or slag in HCC is dependent on the characteristics of the particular material, as discussed here. The presence of fly ash or slag can sometimes be established in hand specimens, whereas establishing the absence of the material or estimating its relative proportion requires more detailed examination of specimens using the petrographic microscope (chapters 12 and 13) or SEM (see chapter 14). Hooton and Rogers (1995) describe quantitative techniques for estimating the percentage of slag and fly ash in HCC by examination of thin sections and XRD analyses. Schlorholtz and Dubberke (1995) used XRF to measure trace elements (Ba and Sr, in this case) to estimate fly-ash content.
As mentioned in chapter 2, reference samples of the common cementitious materials encountered should be maintained in the laboratory for preparation of immersion mounts and thin or polished sections as described in sections 5.4 or 14.3.3. These specimens are examined in either transmitted light (immersion mounts and thin sections) as discussed here or with the SEM (thin and polished sections) as discussed in chapter 14.
This is a one-step procedure:
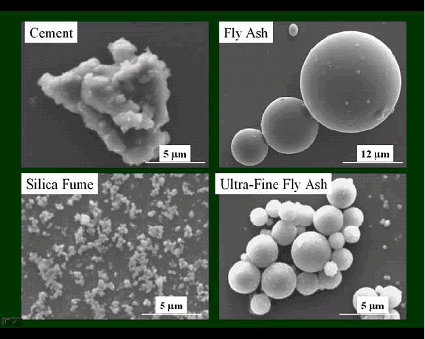
Examine the specimens and describe the particles present: Portland cement is a multiphase material consisting of four major clinker minerals and sulfate. Campbell (1986, 1999) provides an excellent discussion of the microscopic characteristics of portland cements. Figures 132 and 133 show alite (C3S) and belite (C2S) in an immersion mount of portland cement. Crystal morphology and birefringence are keys to identifying the phases in cements and distinguishing them from pozzolans and ground slag. Ground slag and pozzolans are characterized by particle size, shape, and isotropic optical properties resulting from their amorphous nature. Figures 134 through 137 illustrate fly ash (low lime, class F), silica fume, calcined shale natural pozzolan (class N), and ground slag. In crossed polarized light (not shown), the slag and silica fume demonstrate their amorphous character by an essentially total lack of birefringent grains. The bulk of fly ash and natural pozzolans should be amorphous; some crystalline grains exhibiting low birefringence are usually observed. These materials can also be observed using the SEM (chapter 14) where the higher resolving power is particularly useful because of the small particle sizes. In figure 138, SEM images of portland cement, fly ash, an ultrafine fly ash, and silica fume contrast the difference in particle size of these materials.
Figure 132. Immersion mount of portland cement in plane polarized light showing alite crystals.

Figure 133. Immersion mount of belite cluster with darker interstitial aluminate and ferrite.

Figure 134. Immersion mount of fly ash showing spherical particle shape.
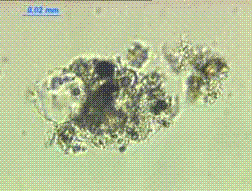
Figure 135. Immersion mount of agglomerate of minute ilica-fume particles.
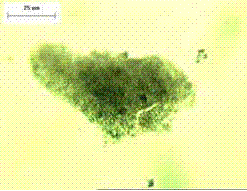
Figure 136. Immersion mount of natural pozzolan (calcined shale).

Figure 137. Immersion mount of ground cement slag.
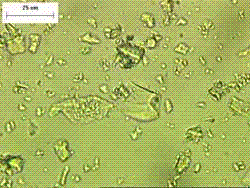
Figure 138. SEM images illustrating the size difference of various cementitious materials.
This is a three-step procedure:
Examine exterior surfaces, broken surfaces, and lapped slices of the HCC: When very young, the pastes containing slag are a characteristic dark bluish green. Mather (1957) investigated the reasons for this color in portland slag cements and concluded that the development of the color varies from cement to cement and is a result of the presence of sulfur compounds in a reduced state (sulfide), the color being lost on oxidation. Regardless of maturity, if these pastes are kept underwater and are not allowed to dry, they will retain this color. As the concrete begins to dry, the surface color fades. If the HCC is sawed or broken while it is only partially dry, it can be seen to be mottled with dark and light places. When it is dry throughout, it will be a uniform light color (see figures 139 and 140). This color is not the same color as ordinary HCC. It is not a gray
Figure 139. Cut surface on HCC containing slag exposed to air for 6 months.
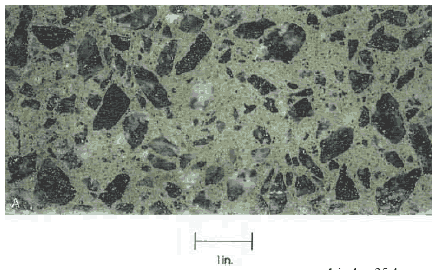
1 inch = 25.4 mm
Figure 140. Interior of beam of HCC containing slag, illustrating the partially dry two-color stage.
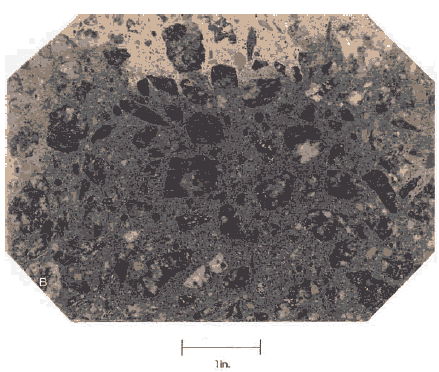
1 inch = 25.4 mm
color, but rather a very light shade of slightly greenish tan or cream. The mottled appearance of an interior surface of slag-bearing concrete of intermediate age is often of concern to field personnel. It has often been thought that the mixing of the HCC was incomplete. In thin sections examined with the petrographic microscope, the dark-colored areas appear to be no different from the faded areas and both types of areas seem to have about the same concentration of slag. If slag was used in the mixture, this mottling is inconsequential and will fade. Slag cannot be detected in mature, fully dried concrete with the stereomicroscope.
This is a four-step procedure:
Figure 141. Thin section of concrete containing slag: At 28 days hydration (note the angularity of the slag fragments).
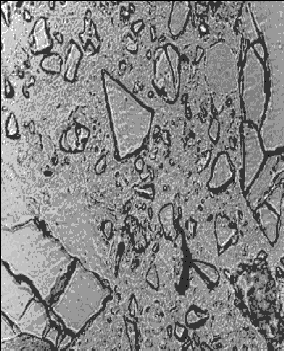
Concentration is 65 percent of cementitious material.
Figure 142. Thin section of concrete containing slag: At 56 days hydration (note the slight rounding of the slag fragments).
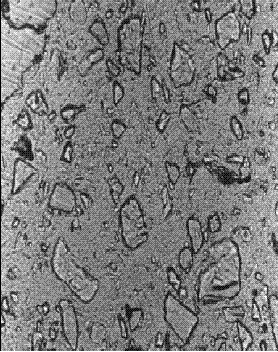
Concentration is 65 percent of cementitious material.
Figure 143. Thin section of concrete containing slag: At 6 months hydration (note the further rounding of the slag fragments)
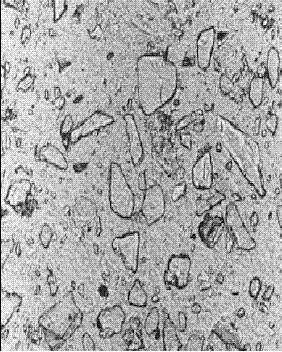
Concentration is 65 percent of cementitious material.
Figure 144. Etched area of lapped surface of concrete containing fly ash.
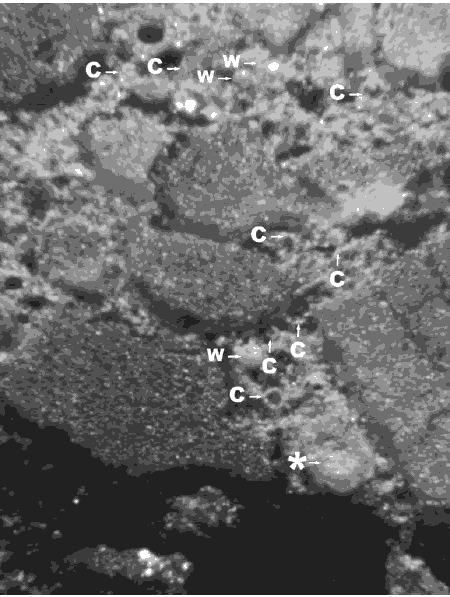
The partial fly-ash particles are indicated by arrows. The particle at the starred arrow is a frothy fly-ash agglomeration. Nearly complete white cenospheres probably filled with froth or a multitude of smaller cenospheres are marked with a "W". These whole cenospheres have been almost completely exposed by the etching procedure; some small whole cenospheres may have been lost during the etching procedure. Cup-shaped portions of cenospheres are marked with a "C".
Figure 145. Fly ash in thin section of HCC, viewed in plane polarized light with a petrographic microscope (note the broken fly-ash particle that is composed of dark and light glass, 100X).
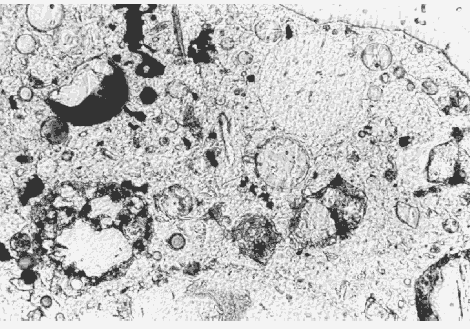
Figure 146. Fly ash in thin section of HCC, viewed in plane polarized light with a petrographic microscope (cenospheres of fly ash filled with smaller cenospheres, 400X).
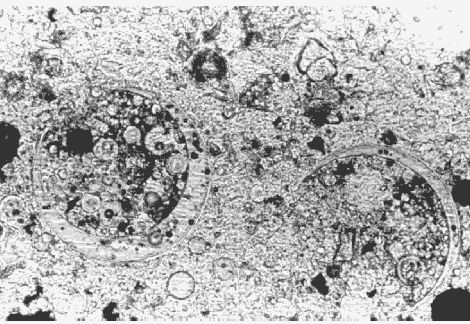
Properly dispersed silica fume has such small particles and is so lacking in birefringence that its presence cannot be detected even in thin sections at 400X. Report that the presence of silica fume cannot be detected by the laboratory. Dry-densified silica fume that is not dispersed or is poorly dispersed will appear as small, dark gray to black particles in reflected light. In transmitted light, they will appear clear, extremely fine-grained, and isotropic. Undispersed silica-fume pellets can cause damage as a result of ASR. Laboratories that have the capability of examining specimens with a SEM can detect and photograph this material.