U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-04-150 Date: July 2006 |
Previous | Table of Contents | Next
The stereomicroscopic examination of the lapped surfaces of the specimen is usually the procedure that controls the course of the analysis of the specimen. If the specimen was submitted for a particular analysis and the petrographer is fairly certain that the other parameters of the concrete do not require investigation (as may be the case when the concrete has been fabricated in a concrete laboratory), the stereomicroscopic examination of the concrete may be omitted. Usually, the petrographer cannot be sure that the examination requested will provide sufficient information to determine all of the possible problems of the concrete submitted and will perform a general stereomicroscopic examination to allow analysis of all of the features of the specimen.
The concerns of the client must be considered throughout any examination of the specimen. However, the observations made should not be confined to these concerns. All of the features of the entire suite of specimens should be inspected and examined in detail. Often, the petrographer is asked to confirm or deny the presence of a particular form of distress, but then finds that there are other kinds of problems present as well.
After any planned quantitative analysis of the constituents (see chapters 6 and 7), five procedures are performed in a general examination with the stereomicroscope, as listed in table 17:
|
In the normal course of events, the preliminary examination (described in chapter 3) is performed and then the specimen is prepared according to the preliminary plan of analysis. If the plan of analysis includes the determination of the air-void parameters, a technician usually performs the procedure. Thus, several days may have elapsed between the original preliminary examination by a petrographer and the stereomicroscopic examination. Occasionally, a petrographer other than the one performing the stereomicroscopic examination may have sketched out the plan of analysis. If the petrographer’s workload is heavy, the time when it is convenient to perform the stereomicroscopic analysis of the specimens may be a few weeks after receipt of the specimen. Therefore, it is usually necessary that the petrographer review the complete history of the HCC and be aware of anything unusual about the design of the mixture and any unusual procedures or occurrences during placement. With this information, the microscopist will be best able to notice and report on any features that might be attributable to new methods, materials, or admixtures, or that might have been caused by incidents that occurred during placement of the HCC. Therefore, the petrographer should review the following: (1) the history of the concrete being studied as reported by the client, either in accompanying documents or orally; (2) any unusual methods of sample preparation that were required; (3) any features noted by the technician during the preparation of the specimens and the air-void analysis; and (4) the results of the air-void analysis.
The stereomicroscope (see figure 20) should be placed on a table or stand that is a convenient height for the microscopist. A variety of microtools (see figure 21), bottles, and droppers to supply water and 10 percent HCl, and a variety of marking equipment to make both temporary and permanent marks on the surface of the lapped slice should be available. A variety of pens; soft, colored pencils; and sticky paper arrows, dots, or other labels for use on the surface of the lapped slice should also be available.
Various pens should be tested on some fine microcracking on an unimportant lapped, oil-free slice of HCC. No particular brand of pen is recommended because inks and pens change as manufacturers find new ways to please the public or economize their operations. The soft or fiber-point disposable pens that have a medium-thick ink work well. While using the stereomicroscope, the microscopist positions the pen on a microcrack and causes a very small portion of the ink to flow onto the visible expression of a crack. If the ink enters the crack (as opposed to beading up on the surface) and by capillary action is drawn a short distance along the crack (without bleeding into the mass of the paste), this small leading portion of the ink, visible with the stereomicroscope, indicates the next direction of the crack. By use of the proper pen to trace the direction of the crack with ink, it is possible to detect and mark microcracks and connections between cracks that cannot be seen against the general paste background at themagnifications of the stereomicroscope. If such pens are available in several colors that will contrast with the HCC, various features can be marked with different colors.
Other pens and pencils are for marking on aggregate surfaces. Light pencil marks are not easily seen on the lapped surface of paste, and heavy pencil marks may damage the surface or fill cavities with graphite or colored flakes.
| NOTE: Most of the features of the lapped slice are easier to see if any shine caused by the lapping oil has been removed from the slice by evaporation (sufficient exposure to room air or overnight treatment in a warm oven). Check the slice during the last stages of the evaporation process to make note of any areas that have absorbed extra oil. Such areas may indicate cold joints, overlay bonds, boundaries of carbonated zones, or transitions between various qualities of the HCC. |
Table 18 is a checklist of the features that should be examined. Figure 73 is a reproduction of a two-page form used by the Ontario Ministry of Transportation. Similar checklists can be found in Van Dam, et al. (2002b).
| Item | Remarks |
|---|---|
| ______ Cross sections of exterior surfaces (quality oforiginal) | |
| ______ Foreign objects | |
| ______ Reinforcing bars, supports, or both | |
| ______ Voids | |
| General Appearance: | |
| ______ Shape | |
| ______ Distribution | |
| ______ Size | |
| ______ Aggregate: | |
| ______ Lithology and mineralogy | |
| ______ Orientation | |
| ______ Aggregate-paste ratio | |
| ______ Distribution | |
| ______ Cracks | |
| ______ Coatings | |
| ______ Aggregate-cement reactions | |
| ______ Paste | |
| ______ Water-cement ratio | |
| ______ Discolored areas | |
| ______ Carbonation | |
| ______ Cementitious materials (portland cement, ground granulated blast-furnace slag, or pozzolans) | |
| ______ Cracks at aggregate bond | |
| ______ Cracks within paste |
The following procedures should be used:
When there is any possibility of the labeling obscuring your ability to see details in the paste (usually the case when using ink to emphasize microcracks), delay any permanent marking and labeling on the paste until you examine the slice for all of the other items on the checklist: When marks and labels can be placed on aggregate surfaces or the labeling can be easily removed, perform such markings as instructed under the various features (concurrent with the general stereomicroscopic examination). Features that may be easily observed with the stereomicroscope may be difficult or impossible to see with the naked eye and are often impossible to record photographically unless they are enhanced by some form of marking or emphasis that can be seen without magnification.
Figure 73. Two-page Petrographic Examination of Hardened Concrete form used by the Ontario Ministry of Transportation—Continued
Courtesy of Christopher A. Rogers, Ministry of Transportation, Ontario, Canada
Examine any cross sections of formed, finished surfaces or wearing surfaces on the slice: The surface of a concrete placement that is not against a form and is generally smoothed and finished by a float, trowel, or texturing device (such as a tine rake or burlap drag) is the finished surface. Ascertain if the paste within a few millimeters of the finished surface is about the same color as the rest of the paste and if the air-void content of the surface layer is not abnormally high. (If the concrete was too stiff to finish easily, the contractor may have added water to the top layer and thus changed the consistency.) A light-colored surface layer (may be no more than 1 mm thick), often with a froth of very fine air voids, may be evidence of the use of excess water during the finishing process or the occurrence of rain. Such a froth will not have much strength and will wear away rapidly; however, fortunately, such zones are usually very shallow and do not greatly affect the durabilityof the placement.
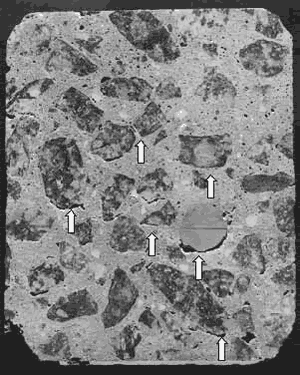
Overworking with the finishing equipment can cause a thin layer at the surface to be exceedingly rich in paste and low in aggregate and air. Excess water will weaken the concrete and sometimes cause cracking and crazing. Excess and misshapen voids, zones of streaked paste, and zones low in air are additional evidence of problems occurring during finishing (see figure 74).
Figure 74. Excess air at surface of concrete (top).

Unless the concrete is old and worn by heavy traffic, the texture of the wearing surface or that of any finished surface should conform to the texture specified. Thin sections that show the profile of the cross sections of the surface can be fabricated (see section 5.3.3). Skid resistance is aided by the production of asperities and the provision of channels in the surface for the escape of water in order to prevent skidding and hydroplaning. Report the condition of the texture on any surface on which traffic moves. Report any differences between the microstructure of the major portion of the paste and the paste adjacent to any exterior surface.
Voids that are within the thin zone that become worked as the concrete is finished and textured can be quite angular. It is only the extremely small voids that maintain their individual integrity when they have been distorted by these procedures. The larger voids are usually broken and thus expelled from the surface; however, many of the tiny ones remain, often angular and distorted. A minimum magnification of 100X is required to discern the presence, shape, and condition of very small voids.
Rarely, retempering (see appendix C) that occurs after the paste has started to harden and the individual voids have developed a “skin$” will cause the majority of the medium and small voids in a concrete to take on an ovoid shape. Most commonly, these voids will have a vertical long dimension. This is a rare condition and is clear evidence of an inappropriate attempt to renew workability.
Figure 75. Clustering of voids apparently caused by excess air-entraining admixture and incomplete mixing (field width is 50 mm).

If the aggregate does not seem to be the aggregate specified in the design of the mixture as reported in the accompanying documentation, contact the client to resolve this inconsistency. Are the specimens received the ones that were meant to be sent? Is the documentation incorrect? Is there some other explanation? To achieve assurance in the identification of the aggregate, it may be necessary to request bulk specimens of the specified aggregate and perhaps specimens of aggregate from other sources. Perform a detailed study of the frequency of occurrence of the various lithologies; determine particle shape, soundness, staining, natural rims, and cleanliness; and study the microstructure and optical properties of these aggregates in thin sections with the petrographic microscope.
Note any deterioration of the HCC that is associated with any particular lithology. Such deterioration includes microcracking; popouts on the surface; reaction rims; and any deposits, particularly those that might indicate an expansive reaction (see chapter 10).
If there is a zone at the top of the placement that is nearly devoid of aggregate, but the aggregate distribution appears normal in the remainder of the placement (as in figure 76), the cause of this distribution is probably the addition of water to the mixture after placement. This water may be added by a rainstorm or possibly even purposefully in order to facilitate placement.
Figure 76. Overwatered concrete: Rain and snow occurring after the concrete was placed caused overwatering near the surface (top). (The aggregate sunk out of the overwatered zone.)

If the aggregate cracks are narrow (or do not seem to separate the aggregate into fragments) or the cracking seems to be in a regular pattern that echoes the surface of the aggregate, the cracking may be a result of the intrinsic cleavage or parting of the aggregate or artifacts of aggregate production. Shaley rocks and monomineralic aggregate particles of particular minerals might be good examples of this type of cracking.
Cracks in the aggregate that do not appear to be a result of the structural nature of the material of the aggregate, that break the aggregate into separate pieces, or that start in the aggregate and extend out into the paste may be caused by reactions and forces to which the concrete has been subjected since hardening. For an example of this, refer to chapter 10.
Because of retempering (see appendix C) or other mixing conditions, HCCs may have areas of low w/cm and areas of high w/cm in the same placement and even in the same specimen or slice. The strength of the HCC will depend on which type of concrete is most prevalent and most continuous through the mass.
Areas of a darker color than the major portion of the paste are probably zones with a high cement content and hence a lower w/cm, and were probably formed before all of the water was added. If sufficiently hydrated, these areas may be very strong. If dry cement is encapsulated in these areas, it will have very little strength and the area will be a zone of weakness. Such areas may indicate retempering (see appendix C) or improper mixing. They occur as two general types: (1) as coatings on and in reentrant angles of the aggregate particles, and/or (2) as knots or blobs of various sizes in the main area of the paste. If such areas are present, mark the slice in such a way that a number of these areas will be noticed in a photograph.
Occasionally, there are zones near the aggregate that are made up of cement that contain little water. These are caused by moist aggregate having come in contact with dry cement and having picked up a cement coating (see figure 77). If these coated aggregates are used in a concrete mixture, the very outside of the cement coating becomes moistened in its new environment; if the coating is thick, the inside can be preserved in a dry condition. If this concrete becomes subject to stress before water permeates the cement shells and hydrates them, these dry cement coatings will be a zone of weakness.
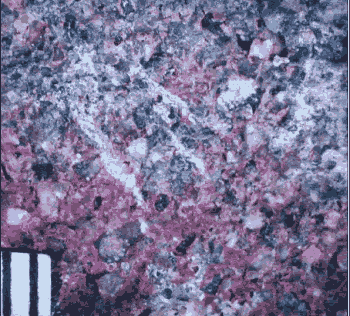
Figure 77. Cement coating on aggregate.
Damp aggregate came in contact with dry cement and picked up a layer that did not become hydrated. This layer is much denser than the zone surrounding the aggregate in figure 54.
Clumps or balls of cement may be caused by water reaching the cement before use or by particular batch procedures. These clumps can be so large and dense that they may be mistaken for aggregates. These areas are usually rounded by the action of the mixer. If a large portion of the cement is formed into balls or coatings on aggregate that survive mixing, the remainder of the concrete will be depleted in cement and will have a high w/cm and be weak.
Variations in the color of the paste may indicate variations in the w/cm (see chapter 9).
If the surface of the slice is rusty looking, it may be because the slice, while it contained a little water, was allowed to sit for many hours on a nonmoving iron lap. Question the support staff concerning this and, if necessary, remind them that laps become pitted by rusting and then must be demounted, resurfaced, or replaced and then remounted on the motor assembly.
Carbonation is a process wherein particular constituents of the HCC paste chemically combine with the CO2 of the atmosphere. Ca(OH)2 within the affected zone reacts with CO2 to form the mineral calcite (CaCO3) and water (H2O). Some loosely held calcium ions in the calcium silicate hydrates of the cement paste might alter to form fine crystals of the carbonate minerals, mostly calcite. When the paste is treated with acid (see section 5.2.3), the CaCO3 dissolves with effervescence. The uncarbonated paste is very soluble in the acid, and often the carbonated zone (now with the calcite removed) appears porous, but remains higher than the etched uncarbonated paste. After acid treatment, the portion that had been carbonated is usually cream or white in color. There is often a ridge of especially high relief at the line of contact between the carbonated and uncarbonated areas (see figures 45 and 46).
If the etching does not clarify the difference between carbonated and uncarbonated paste, fabricate thin sections for examination with the petrographic microscope. The high birefringence and the fine crystallite size of the products of carbonation, when viewed through crossed nicols, will indicate the presence of this alteration of the paste (see figures 156 through 160).
Figure 78. Fly-ash particles on the surface of a lapped slice of concrete.
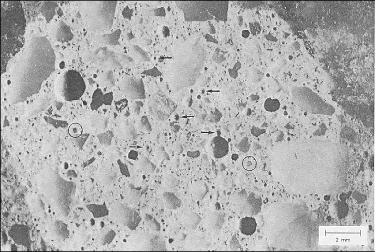
These particles can be recognized by their glassy interiors. Lighter colored fly ash is marked with arrows; the black fly ash is encircled. If the slice had been etched, more fly-ash particles would have been detected.
These bond cracks are more apt to occur in mixtures with a high w/cm. The layer of water attracted to the aggregate creates an interface transition zone primarily filled with calcium hydroxide. Pockets of calcium hydroxide are not as strong as calcium silicate hydrate, and thus weak zones are created. In very old porous concrete subject to the action of running water or groundwater, calcium hydroxide in the ITZ may leach out and leave an empty boundary crack.
Conflicting evidence exists concerning the prevalence of bond cracks in concrete containing expanded aggregate. In some, the aggregates seem to have developed a surface that repels water and makes a tight bond with the cement paste. In others, they seem to develop as many, if not more, bond cracks than do quartz gravel aggregates (see appendix D). Be conscious of the condition of the ITZ and for any means of explaining poor paste-aggregate bond. Voids or cracks at the ITZ can be serious zones of weakness and contribute significantly to permeability (see figure 79).
Microcracking and similar fine details are very difficult to see on a rough surface and usually cannot be observed on anything but a well-prepared finely lapped surface. Sometimes, the microcracking seen on a finely lapped interior surface will indicate the general location of the cracks on a nearby rough exterior surface. The thinner the slice, the more relationship will be found between the nearly invisible surface microcracks and the microcracks found on the lapped surface (see figures 81 and 82).
The field of view seen through a microscope is very limited, and it is very difficult to construct a mental image of how one feature relates to another and be constantly aware of which portion of the specimen is under observation. A crack seen in one view may be followed across the specimen until it disappears, gets to the edge of the specimen, or abuts another feature; however, the overall relationship of all cracks to each other can be determined only by marking the cracks with ink and examining the specimen without magnification.
The procedure for marking microcracks in the paste is as follows: While viewing the slice with the stereomicroscope, carefully mark the microcracks with a pen. Use the movement of the ink as it follows the minute cracks by capillary action to find all the connections between the microcracks (Walker, 1988). The visibility of microcracks depends a great deal on the angle of the illumination and the angle of viewing. As the specimen is turned and moved under the microscope, more and more cracks become visible. The mind and eyes become concentrated on the view in the microscope, and the hands become totally engaged in the tasks of marking and moving the slice of HCC. Thus, a sort of hypnotic state is created that leaves no part of the mind free to relate the area being marked to a larger view of the slice as a whole. Many operators find that they are quite startled when the crack being marked extends to the edge of the slice and the crack falls into oblivion.
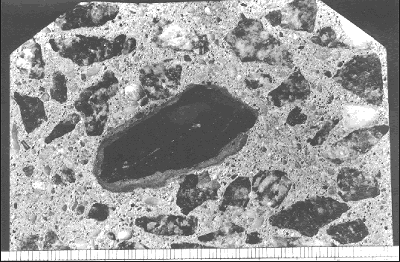
Figure 79. Cracks at the bond between the aggregate, rebar, and paste (see arrows).
In this instance, the bond cracks occur most frequently on the underside of the aggregate and, therefore, can probably be attributed to bleeding or poor consolidation.
Figure 80. Cracks typical of damage caused by cycles of freezing and thawing.

Such cracks occur in non-air-entrained concrete (cracks are emphasized with ink).
Figure 81. Microcracks: Smoothly lapped surface (A) with ink-marked microcracks.
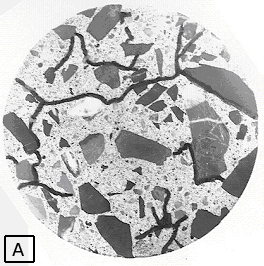
Figure 82. Microcracks: Wearing surface (B) near figure 81.
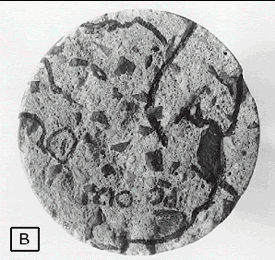
The crack pattern seen in figure 81 in surface (A) was used to guide the finding of the cracks in surface (B) the reverse side of the same specimen, figure 82.
This method can be very time-consuming if there is a great amount of microcracking. Moving the slice of concrete under the microscope in an effort to check and mark the cracks and the connecting cracks of the total area can be a seemingly endless task. To get the work done in a reasonable amount of time, a possible procedure is to mark off a randomly selected portion of surface on each slice to be examined and compared. The size of the portion should be governed by the uniformity of the cracking and its frequency (50 by 50 mm might seem like a lot of area when magnified). Do not use a pen to mark off the portion. Ink may follow capillaries into the area to be examined. Use a narrow adhesive tape or a soft pencil in a bright color. The marking of the cracks and other important features can then be performed on these smaller portions of the surfaces (see figures 83 and 84).
Once the microcracks are marked, the patterns of microcracking will be visible to the unaided eye and varied HCCs can be compared, and photographs of the microcrack pattern may be prepared and used as evidence of the true condition of the HCC, as shown in figure 85.
If the placement has been overlaid with another material, such as latex concrete, the client may ask if there is a crack at the bond line. In such a case, study a vertical lapped surface and mark all microcracks with ink. Often, any separation between the overlay and the substrate concrete is not at the bond line, but is in the weaker material of the substrate (see figure 86). This just-below-the-bond cracking is often discontinuous; however, cycles of freezing and thawing and traffic may well cause complete delamination. This type of cracking usually indicates that the surface preparation techniques used were inadequate. The use of a milling machine to remove a layer of concrete will leave a damaged substrate surface that will impede bonding of the overlay. This milled surface layer should be removed by shotblasting or a similar technique before the overlay is applied.
Figure 83. Finely lapped surface of beam tested for resistance to cycles of freezing and thawing.

Figure 84. Finely lapped surface of beam tested for resistance to cycles of freezing and thawing: Mixtures in figures 83 and 84 were identical except that the mixture shown here contained an experimental admixture.
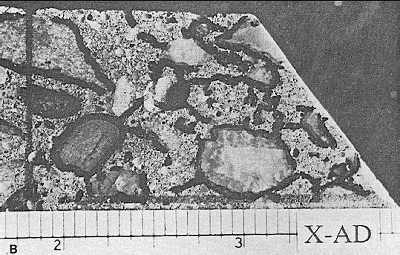
Figure 85. Lapped surface of slice of concrete containing prestressing strand.
With the stereomicroscope, the specimen could be seen to contain many fine cracks; however, the relationship between all of the cracks and the reinforcing cable could not be noted until the cracks had been marked with ink and the crack pattern examined without magnification. The field of view (the portion of the slice) seen at the magnification necessary for observation of the fine crack system is about the size of one wire of the cable.
Figure 86. Cracking just below the bond in the concrete deck with latex-modified concrete overlay.
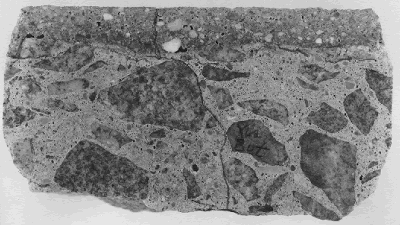
Notice that the vertical crack in the substrate continues through the latex concrete and is expressed at the wearing surface.
All the notes produced during the examination of the slice should be reviewed, and all of the marks that are intended to be visible in photographs should be made permanent and clear enough to be recorded in a photograph. If they are not, they should be enhanced.
| CAUTION: Most inks begin to fade after they have been in contact with HCC paste for a few days. Therefore, obtain any photographs promptly and make any necessary assessments of the condition of the concrete within a day or two. It is not known whether this fading is more rapid on young or old concrete or if it is a result of the ionic movement of the ink (sinking into and being distributed in a dilute condition throughout the HCC) or a chemical reaction. |
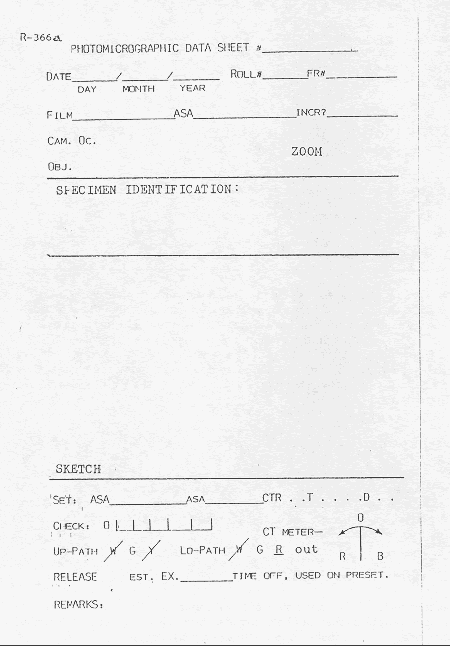
Each exposure should be recorded in a notebook or file expressly designed for such data. Data that will aid in improving future photographs should be included: (1) record the illumination and the adjustments to the light meter or shutter control and (2) record the ground cloth and background when appropriate. A system for identifying all negatives should be devised and this identification should be recorded with the negatives, on any archive prints, and in all notes concerning the photographs or specimens. Figure 87 is a sample of a sheet used in the VTRC stereomicroscopy photograph notebook.
The photographs may include the entire slice, or small areas of the slice may be recorded as enlarged by a camera lens or enlarger. Such photographs should be included in any important final report; they will be invaluable in any legal controversy. A permanent record should be made in the file regarding the roll and frame numbers or photographic file numbers.
Important features that are visible only with magnification should be recorded in photomicrographs so that they can be easily discussed with the client. A record of their appearance should be included in the data file on these specimens. Such pictures can be taken with a camera on the upright port of the stereomicroscope (see figure 19).
At various stages throughout an examination, it may be beneficial to use stains or spot tests to develop information on the chemical nature of the material in the specimens. These tests can be used on freshly sawed or fractured surfaces, lapped surfaces (either slabs or thin sections), or material plucked or otherwise extracted from specimens. The most common of these is the use of phenolphthalein to indicate the pH of the paste (figure 88). These tests have their origins in basic qualitative chemical analysis (Feigl, 1939; Jungreis, 1997), relying in most cases on ion-exchange principles to detect the presence of particular elements. As such, they do not positively identify the character of the tested material (e.g., as alkali-silica gel), but rather provide information regarding the chemical composition of the material (the presence of sodium or potassium) that aids in its identification and origin.
Two such tests have been identified as useful for alkali-silica reactivity (ASR) products?one for sodium using a uranyl acetate solution (Stark, 1990), the other for potassium using a sodium cobaltinitrite solution (Guthrie and Carey, 1997, 1999). The uranyl acetate test is detailed in section 10.2.3 and is an annex to ASTM C 856. It uses the principal of ultraviolet fluorescence of the uranyl ion and consequently requires a source of electric power and an opaque viewing box (portable darkroom) for use in an outdoor environment. The potassium spot test is simpler since potassium cobaltinitrite has a strong yellow color in normal light. It is also more environmentally friendly than the uranyl acetate test (Guthrie and Carey, 1997, 1999). It is important to emphasize that positive indications in these tests merely signify the presence of the particular ion in an exchangeable form in the substance being tested. Poole, McLachlan, and Ellis (1988) report the use of cuprammonium sulfate for detecting ASR products.
Figure 87. Sheet used in VTRC stereomicroscopy photograph notebook.
If the uranyl acetate test is used, care must be taken to follow all the safety precautions described. A fresh concrete surface free of laitance and carbonation is required. In the laboratory, a fresh surface can be sawed or produced by breaking the specimen with a sledgehammer or in a trimmer (figure 11). In the laboratory, the uranyl acetate-treated specimen is viewed in ultraviolet light in a dark place (closet or darkroom).
Any powdered concrete is rinsed from the surface. The uranyl acetate solution is sprayed on the surface, allowed to react for 5 min, and then rinsed away. The concrete is examined for fluorescent gel with the ultraviolet light (figures 89 and 90).
Figure 88. Specimen treated with phenolphthalein (high-alkalinity, uncarbonated paste stains pink; carbonated paste does not stain).
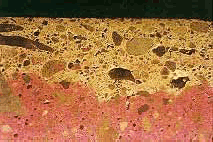
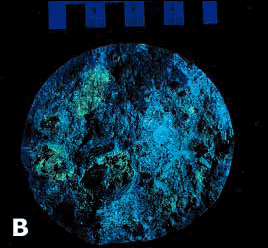
Figure 89. Specimen from retaining wall with destructive ASR treated with uranyl acetate: Ordinary light.

Figure 90. Specimen from retaining wall with destructive ASR treated with uranyl acetate: Ultraviolet illumination causes silica gel to fluoresce (darkroom photograph).
For the sodium cobaltinitrite test, the freshly fractured or polished surface is dampened with water and then the test solution is applied. Excess solution is rinsed from the specimen with water after several minutes of treatment. Observe the treated surface for evidence of the yellow precipitate (figure 91).
Figure 91. Polished specimen treated with sodium cobaltinitrite, which stains the potassium-bearing compounds yellow.

Sulfate compounds often exist as cryptocrystalline clear to white secondary deposits in voids or cracks and can be distinguished using a barium chloride, potassium permanganate solution (Poole and Thomas, 1975). If sulfate ions are present, BaSO4 will precipitate, trapping permanganate in the crystal structure, thus staining purple (figure 92). Ray (2000) describes the use of Alizarin Red S solution that stains ettringite bright orange, whereas thaumasite, another sulfate mineral (containing carbonate) of similar appearance, stains pink to red. Alizarin Red-S solution also has use in differentiating carbonate minerals by their chemical makeup (Heinrich, 1965; St. John, et al., 1998).
Figure 92. Fractured surface treated with BaCl-KMnO4 (secondary deposits containing sulfate are stained pink).