U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-06-073 Date: July 2006 |
Previous | Table of Contents | Next
This chapter reviews research to date on using lithium compounds to control ASR. Included are discussions on the basic chemistry and production of lithium compounds, and the mechanisms by which lithium compounds control ASR. It also provides a critical review of various laboratory evaluations of lithium, including the effects of lithium on ASR and on other concrete properties. Significant emphasis is placed on combining lithium technology with other more traditional materials, including fly ash and slag.
The information and data presented in this chapter are limited to laboratory evaluations using lithium compounds. Chapter 4 summarizes various case studies of actual field applications of lithium compounds to control or mitigate ASR in new and existing concrete structures. Guidelines are provided later in this report on how to efficiently test, specify, and use lithium compounds.
Lithium is an alkali metal found in Group IA of the periodic table and has an atomic number of 3. Lithium is a soft, silver-white metal and is the lightest dense metal, with a density about half that of water (0.53 g/cm3). Lithium is a very reactive metal because of its tendency to expel its outer electron (it has a valence of +1). It does not occur freely in nature, but rather it is bound in stable salts or minerals.
The most common sources of lithium are pegmatite rocks, which are coarse-grained granites composed of quartz, alkali feldspar, and possibly mica, and salt brine lakes. The main lithium-bearing minerals include spodumene, petalite, amblygonite, lepidolite, and eucrypite; the chemical compositions of these minerals are shown in Table 4. The table also includes the location of major mineral deposits, although other significant deposits have also been identified worldwide.
| Mineral | Formula | Locations of Deposits (in alphabetical order) |
|---|---|---|
| Spodumene | Li2O·Al2O3·4SiO2 | Australia, Brazil, Canada, China, Russia, United States |
| Petalite | Li2O·Al2O3·8SiO2 | Australia, Brazil, Namibia, Russia, Sweden, Zimbabwe |
| Amblygonite | LiAl(PO4)(F,OH) | Brazil, Canada, Mozambique, Namibia, Rwanda, South Africa, Surinam, Zimbabwe |
| Lepidolite | K2(Li,Al)5-6{Si6-7Al2-1)20} (OH,F)4 | Brazil, Canada, Namibia, Zimbabwe |
| Eucryptite | Li2Al2O3·2SiO2 | Zimbabwe |
Of the minerals shown in Table 4, spodumene is the most common lithium ore. However, because extracting lithium from spodumene is an energy-intensive process (requires heating to approximately 1100 °C), most lithium production has shifted in recent years to the use of subsurface brine deposits (Ober, 2000). Lithium is extracted from brine sources by solar evaporation of concentrated brine, a process that is significantly less expensive than extracting lithium from ore deposits. Lithium is obtained from brine deposits found mainly in North and South America-Chile and Argentina are major sources (Ober, 2002).
Lithium carbonate (Li2CO3) is the most important lithium compound produced from brine and ore deposits, and in most cases, other lithium compounds require Li2CO3 as a feedstock for further processing (Ober, 2002). In addition to Li2CO3, other commonly produced lithium compounds include lithium nitrate (LiNO3,) lithium hydroxide and monohydrate (LiOH, LiOH·H2O), lithium chloride (LiCl), lithium fluoride (LiF), and lithium sulfate (Li2SO4).
The use of lithium compounds to control expansion due to ASR was first reported by McCoy and Caldwell (1951). They conducted a comprehensive investigation on the potential use of chemical admixtures to prevent or minimize ASR-induced expansion and damage. More than 100 different compounds were included in this study, including metallic salts, acids, oils, organic chemicals, proteins and proprietary admixtures. McCoy and Caldwell conducted a series of ASTM C 277 mortar bar tests (using Pyrex glass as the reactive aggregate) and reported that the most promising candidates in reducing ASR expansion were lithium compounds (LiCl, Li2CO3, LiF, Li2SiO3, LiNO3, and Li2SO4), which essentially eliminated expansion after 8 weeks storage at 38 °C, provided they were used in sufficient quantity.
For about 40 years after McCoy and Caldwell published the findings of their study, only a few studies were conducted on the effectiveness of lithium compounds to control ASR. In the past 10 years, however, there has been resurgence in the interest in lithium-bearing compounds. This is reflected in an increase in scientific publications on the topic and field applications of lithium-based products.
As previously mentioned, McCoy and Caldwell (1951) used mortar bars prepared with high-alkali cement and reactive glass aggregate to demonstrate the effectiveness of lithium additives in reducing expansion associated with ASR. These results and the results of more recent research (Chatterji, 1987; Sakaguchi, et al., 1989; Stark, 1992; Lumley, 1997; Ramachandran, 1998; Diamond, 1999; Thomas, et al., 2000, Collins, et al., 2004; Tremblay, et al., 2004), that show a reduction in expansion by ASR in the presence of lithium-containing additives, have generated much interest in using these compounds in concrete structures. While it is known that some lithium additives are effective at reducing expansion in concrete affected by ASR, the mechanism or mechanisms by which these additives reduce expansion are not clearly understood. Without understanding the control mechanism, it may be difficult to predict the effectiveness of a particular chemical additive or dosage, or to predict the duration of its control. However, several mechanisms have been proposed to describe the effect of lithium, including:
Several researchers have proposed that a less expansive or nonexpansive product may form during alkali-silica reaction in the presence of lithium. Stark (1992) proposed that during the alkali-silica reaction, in the presence of a sufficient concentration of lithium, a lithium-alkali (and possibly calcium) silicate forms that has little or no capacity for expansion. Because the research suggests that a minimum lithium threshold content is required to inhibit expansion, Stark reasoned that the product must contain a minimum proportion of lithium to be nonexpansive. Similarly, Diamond and Ong (1992) showed that, as the amount of lithium present in the gel product increased in proportion to the amount of sodium and potassium present, mortar bar expansion decreased, confirming the work of Stark (1992).
Lawrence and Vivian (1961) suggested that a lithium silicate forms by ASR in the presence of lithium, and that this product is less soluble and more stable than the ASR product in the absence of lithium. Due to its stability, it was proposed that the resulting LiOH silica complex may form an insoluble surface layer, protecting silica from further attack by other alkalis (Lawrence and Vivian, 1961).
Lawrence and Vivian (1961) also showed that silica gel tended to be less reactive with NaOH with increasing concentrations of LiOH, to 2N LiOH equivalent in 2N NaOH equivalent solution. By measuring lithium and alkali concentrations in expressed pore solutions from mortar bars, Sakaguchi, et al. (1989) found that the concentration of lithium decreased with time, while alkali concentrations remained nearly constant. In the absence of lithium, the alkali concentration of the expressed pore fluid decreased over time, suggesting that the lithium-silica reaction is more favorable than the sodium-silica or potassium-silica reaction in mortar bars. According to this theory, the formation of a nonexpansive lithium-containing product would be favored over the formation of a more expansive product containing relatively greater concentrations of the alkalis sodium and potassium.
Others (Chatterji, 1987), however, disagree, stating that the reaction of silica with sodium is favored over the reaction of silica with lithium. In a system containing Na+, K+, and Li+, the alkalis compete for adsorption at negatively charged sites on the silicate surface. Since adsorption affinity increases with bare cation radius, it is expected that sodium adsorption will be preferential to lithium adsorption, in disagreement with Sakaguchi (1989). Kurtis, et al. (1998; 2003), however, have proposed that strong field exchange behavior, where cations are in direct contact with a surface, may account for the preference of the alkali-silica reaction gel for Li+ as compared to Na+ and K+, as described by Sakaguchi (1989).
Ramyar, et al. (2004) reported the results of a study to determine the chemical and morphological characteristics of reaction products from mortar bars, with/without lithium (LiF, Li2CO3 used in the mixing water) or fly ash, subjected to ASTM C 1260 test conditions. The use of fly ash and lithium-based admixtures resulted in significant reduction in mortar bar expansion, while expansion seemed to stabilize after 14 days for Li-bearing mortar bars. Also, observations using scanning electron microscopy (SEM) and energy dispersive X-ray microanalysis confirmed that the morphology of the crystalline reaction products was changed by the use of Li-bearing admixtures.
Mei, et al. (2004) studied the properties of alkali-silica reaction products formed from the reaction of silica particles in LiOH, KOH, or NaOH test solutions. They found that the water absorption capacity of the reaction products, when further exposed to relative humidity ranging from 25 to 100 percent, always increased as follows: KOH > NaOH > LiOH. The authors also evaluated the expansion of concrete microbar incorporating a highly reactive zeolitized perlite aggregate after autoclave treatment (at 150 °C) for a few days in water, LiOH, KOH, or LiOH+KOH solutions. The authors found that the use of LiOH (in the bar and the soak solution) can inhibit ASR expansion and cracking. Reaction rims surrounding the reactive silica and secondary reaction products were observed in both systems; however, "textured" (approximately crystalline) lithium-based silicates were formed when LiOH was present in the system. The authors proposed that since the charge density of Li+ is much higher than that of K+, the ion binding force of Li-Si is stronger than that of Li-K, thus forming lithium silicates (L-S-H) of nonexpansive character surrounding and protecting the reacting silica from further deleterious reaction with K ions.
Kawamura and Fuwa (2003) reported that adding lithium to mortar mixtures decreased the CaO/SiO2 ratio in ASR gel. This ratio was found to decrease with increasing lithium dosages until a threshold lithium concentration was reached, above which the gel composition became essentially insensitive to further increases in lithium concentration. The link between the CaO/SiO2 ratio in gel products and expansion was not clearly established in this study, but the direct impact of lithium on gel composition may eventually shed light on expansion mechanisms.
In examining the effect of various alkali-hydroxides on silica dissolution rate, Lawrence and Vivian (1961) found that the dissolution rate increased in this order:
KOH > NaOH > LiOH (4)
Wijnen, et al. (1989) found that the rate of silica dissolution decreased in a similar order and proposed that this rate decreases with increasing hydrated ion radius of the alkali metal cations in solution surrounding a silicate surface. Considering lithium, sodium, and potassium, the rate of silica dissolution would, then, be slowest in the presence of lithium, which has a larger hydrated ion radius than sodium which is, in turn, larger than potassium. Chatterji, et al. (1987) proposed that the size of the hydrated ion radius was important in determining the extent of chemical reaction during alkali-silica reaction, supporting research findings that degree of chemical reaction increased from lithium to sodium to potassium.
These results suggest that lithium may act to decrease the rate of silica dissolution, which would then limit the rate of product formation and potential for expansion. Over time, however, the concentration of dissolved silica for each alkali-hydroxide concentration examined by Lawrence and Vivian (1961) approached the same value, independent of alkali type, suggesting that lithium may reduce the rate of dissolution, not the solubility of silica. In examining silica gel in model pore solution, Collins (2002), however, found that silica concentration in solution decreased with increasing lithium concentration over a period from 1 hour to 28 days. The results of Collins (2002) may demonstrate, again, the significance of dosage. If, as Sakaguchi's (1989) results suggest, the lithium reaction with the silica or ASR gel is preferential to the reaction with sodium and potassium cations, local concentrations of lithium near the silica may decrease the rate of dissolution, effectively decreasing the rate of formation of the expansive gel.
Qi and Wen (2004) proposed that lithium hydroxide reduces the dissolution of silica and results in a different morphology of reaction products. The authors reported that a layer of lithium silicate forms on the surface of reactive aggregates, thereby reducing subsequent dissolution. This work was based on observing gel products and measuring expansions in solution testing (sol-gel test) in the presence and absence of lithium hydroxide. It was shown that [Li]/[Na+K] ratios in excess of 0.8 were required to reduce the expansion of opal in sol-gel testing by approximately 90 percent.
Collins, et al. (2004) analyzed slurries of silica gel and alkali solution at various ages to determine changes in concentration of solution phase species (Si, Na, Ca, and Li). Quantitative analysis showed that sodium and lithium were bound in reaction products found within the slurries. It was also noted that lithium may have been preferentially bound over sodium in at least one of the reaction products. From this research, it appeared that lithium additives either decreased silica dissolution or promoted precipitation of a silica-rich product (some of which may be nonexpansive) as the dissolved silica concentration decreased with increasing dosage of lithium nitrate or lithium chloride. Slurries prepared with LiCl and LiNO3 evidenced a decrease in silica dissolution with a corresponding increase in additive amount, whereas slurries prepared with LiOH showed an opposite trend. The increase in silica dissolution in the case of LiOH did not translate to an increase in expansion in mortar bars prepared with LiOH. Therefore, it was proposed that expansion due to ASR in the presence of lithium was most likely not dependant upon the quantity of dissolved silica. The authors purport that the reduction in expansion when lithium is present may be due to the formation of a nonexpansive lithium-silicate complex.
Based upon microscopy, elemental analysis, and surface chemistry principles, Kurtis, et al. (1998, 2003) have suggested that, in addition to decreasing the rate of silica dissolution, lithium may limit repolymerization of dissolved silica species into a gel, effectively reducing the potential for expansion. Using x-ray microscopy to examine the reaction of silica in model pore solution in the presence and absence of lithium, Kurtis, et al. (1998, 2000, in press) observed that significantly more gel product formed in samples containing no lithium than in those containing lithium. Companion results from elemental analysis showing a decrease in silicon concentration in solution indicated that the presence of lithium decreased silica dissolution to some extent, but the concentration of silicon in solution in the presence of lithium was on the order of the concentrations observed in the absence of lithium. These results suggested that the differences in behavior observed with lithium may be due more directly to changes in the amount of product formed rather than in the degree of reaction. Kurtis and Monteiro (2003) also propose that lithium yields a reduction in surface charge density of ASR gel.
If, as the research of Sakaguchi (1982) suggests, adsorption of Li+ is favored over Na+ and K+ adsorption, a physical mechanism for preventing gel repolymerization may exist. Iler (1956) postulates that the highly hydrated lithium ions are not adsorbed as near to the silicate surface as a cation which has a smaller hydrated radius, such as sodium or potassium. Thus, Kurtis, et al. (1998, 2000, in press) proposed that the net repulsion between the silicate particles remains high in the presence of lithium. As a result, it is theorized that when lithium is present in sufficient concentrations, repolymerization into a potentially expansive gel does not occur. The effect of the lithium should, then, depend upon its relative concentration in the solution as well as the favorability of the silica-lithium reaction.
Prezzi, et al. (1997) proposed the use of the electrical double layer (EDL) theory to explain the expansion of the ASR gel, and the theory was extended to describe a proposed mechanism by which chemical additives, including lithium salts, may inhibit expansion (Prezzi, 1998). Applying these principles, the ASR gel is assumed to act as a colloid composed of negatively charged particles. According to the theory, swelling of the gel is attributed to double-layer repulsion effects between the colloidal particles. According to EDL theory, the valence and hydrated radius of cations in the colloid are important factors in determining the expansion of the gel. The double-layer theory predicts that an ASR gel containing larger concentrations of cations with larger valences will exhibit less expansion. That is, higher proportions of trivalent (e.g., Al3+) and bivalent (e.g., Ca2+) cations relative to monovalent cations (e.g., Na+, K+, and Li+) should result in less expansive gels. Results from a series of mortar bar tests performed by Prezzi, et al. (1998) agree with double-layer predictions according to cation valence. These tests showed expansion increasing with cation charge in the order:
Al+3 < Ca+2 < Mg+2 < Li+2 < K1 < Na 1 (5)
However, it is important to recognize that the size of the hydrated ion radius also will affect expansion as predicted by EDL theory. As stated previously, the radius of the hydrated lithium ion is larger than those for sodium or potassium. Therefore, EDL theory predicts that lithium will produce greater expansion compared to these other monovalent cations, not reduce expansion, as is generally evident. Therefore, additional mechanisms may need to be considered to explain the reduction in gel expansion associated with the use of lithium additives.
According to Prezzi (1997, 1998), a decrease in surface charge density (-s) effectively reduces the pressure (ΔP) generated by ASR gel expansion:
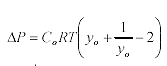 (6)
(6)
Where: Co is the bulk electrolyte concentration
R is the molar gas constant
T is the absolute temperature and
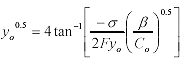 (7)
(7)
Where: F is the Faraday constant
β = 1.084x1016m/mol at 25 °C
σ is expressed per unit area.
The presence of bivalent and trivalent cations provided by chemical salts has been theorized to lower surface charge density of the ASR gel as compared to systems where more monovalent cations are present. Rodrigues, et al. (2001; unpublished) have performed potentiometric titrations to examine how the surface charge density of silicates, including ASR gel, is affected by the presence of various chemical salts, including LiCl. Their work showed that in a system with both sodium and lithium ions present, the surface charge density of opal (2001) and alkali-silica gel (obtained from an ASR-affected dam) (unpublished data) were decreased in the presence of LiCl, as compared to NaCl. A decrease in the repulsive forces between colloidal particles of ASR gel in the presence of lithium would reduce expansive pressure generated by swelling of the gel. However, Rodrigues (2001) also observed that KCl produced an even greater reduction in surface charge density than LiCl. These results do not coincide with the findings of Prezzi, et al. (1998) described above and suggest that further research is necessary.
This section reviews research to date on using lithium to control ASR, beginning with the initial investigation by McCoy and Caldwell and proceeding to recent studies. Because most of the laboratory (and exposure site) studies have dealt with lithium compounds as an admixture, only limited information is available on post-treating hardened concrete with lithium to mitigate further expansion.
Many laboratory studies have focused on the use of lithium compounds to control ASR, some of which are discussed in section 3.3.2, as related to proposed mechanisms. The studies span more than 50 years and have used different test methods, lithium compounds, cementitious materials, and aggregates, making it somewhat difficult to compare one study directly to another. However, general comparisons are possible and trends in test results can be identified. These then can be synthesized and incorporated into guidelines and recommendations for efficiently testing, specifying, and using lithium to control ASR. Of particular interest in the review that follows is the documented dosage of lithium required to control ASR for aggregates of different levels of reactivity and for mixtures containing SCMs. Information is also provided on recommended methods of assessing lithium compounds in mortar and concrete, expanding upon the information provided in Chapter 2 on testing methodologies. At the end of this section, the main findings from the various studies will be summarized and incorporated into specific guidelines for using lithium in new concrete.
As previously discussed, McCoy and Caldwell (1951) were the first researchers to identify lithium compounds as effective admixtures in controlling ASR. Their study included the use of LiCl, Li2CO3, LiF, Li2SiO3, LiNO3, and Li2SO4 at various dosages. Testing was performed according to ASTM C 227, with Pyrex glass as the reactive aggregate. Each of the lithium compounds was found to be effective in minimizing expansion, provided that a high enough dosage was used.
Table 5. Effects of Lithium Compounds on Mortar Bar Expansion (From McCoy and Caldwell, 1951).contains the expansion data (at various ages) for mortar bars containing different lithium compounds, where the dosages listed are based on mass of cement. A more convenient and useful method of displaying this data is to express expansion as function of the lithium-alkali molar ratio. Figure 11. Relative Expansion of Mortar Bars Containing Lithium Compounds (After McCoy and Caldwell, 1951). shows the relative expansion of mortar bars containing lithium to a control without lithium (where a value of 1.0 reflects no effect on expansion), plotted against the lithium-alkali ratio (which is equal to the moles of lithium divided by the moles of sodium plus potassium). As the amount of lithium in mortar increased, the relative amount of expansion decreased. The data indicated that a molar ratio of lithium to alkali of 0.74 or above was sufficient to suppress expansion efficiently.
| Lithium Salts | % Addition (by mass of cement) | % Reduction in Expansion | |||
|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 6 weeks | 8 weeks | ||
| Lithium Chloride | 0.50 | 75 | 43 | 34 | 34 |
| Lithium Chloride | 1.00 | 90 | 91 | 90 | 88 |
| Lithium Carbonate | 0.50 | 89 | 68 | 67 | 62 |
| Lithium Carbonate | 1.00 | 94 | 94 | 93 | 91 |
| Lithium Fluoride | 0.50 | 92 | 92 | 89 | 82 |
| Lithium Fluoride | 1.00 | 97 | 99 | 98 | 98 |
| Lithium Nitrate | 1.00 | 81 | 72 | 31 | 20 |
| Lithium Sulfate | 1.00 | 88 | 72 | 53 | 48 |
Figure 11. Relative Expansion of Mortar Bars Containing Lithium Compounds (After McCoy and Caldwell, 1951).
After the early findings of McCoy and Caldwell, it was approximately 40 years before research on controlling ASR with lithium compounds continued in earnest. A series of studies were initiated in the late 1980s and early 1990s, and more recent studies have been conducted or are still in progress. Several of these studies are reviewed briefly below. Most of these studies have used ASTM C 227 as the method of testing lithium, but some of the more recent investigations have used the CPT (ASTM C 1293) and a modified version of the accelerated mortar bar test (ASTM C 1260).
As described in section 3.3.2, Sakaguchi, et al. (1989) proposed that the formation of a nonexpansive lithium-containing product would be favored over the formation of a more expansive product containing relatively greater concentrations of the alkalis, sodium and potassium. Their study included the use of LiOH·H2O, lithium nitrite (LiNO2), and Li2CO3 in mortar bars containing Pyrex glass, and LiOH·H2O in mortar bars containing a reactive pyroxene andesite sand from Japan. All of the lithium compounds were effective in reducing expansion, with increasing lithium dosages resulting in decreased expansion. For the tests performed using LiOH·H2O with the reactive aggregate, the reductions in expansion are shown in Table 6. A molar ratio of 0.9 was sufficient to completely suppress expansion.
| Dosage of LiOH·H2O (by mass of cement, %) | Molar Ratio [Li]/[Na+K] | Expansion after 1 Year (%) | Relative Expansion Compared to Control (%) |
|---|---|---|---|
| 0.00 | 0 | 0.88 | 100 |
| 0.27 | 0.3 | 0.86 | 97 |
| 0.55 | 0.6 | 0.48 | 55 |
| 0.83 | 0.9 | 0.05 | 6 |
| 1.11 | 1.2 | 0.04 | 5 |
Ohama, et al. (1989) investigated the use of LiOH·H2O, LiF, and Li2CO3 in mortar bars containing opaline amorphous silica as the reactive aggregate. The bars were cured for 1 day at 20 °C and 100 percent RH, then subjected to autoclave curing at 128 °C under a pressure of 2.5 kgf/cm2 for 4 hours, after which the bars were cooled back to 20 °C and measured for expansion. Li2CO3 only slightly reduced expansion, whereas LiF and LiOH·H2O at 0.5 percent and 0.7 percent (based on mass of cement), respectively, reduced the expansion to about half of that of the control. Because of the extreme conditions typical of high-temperature autoclaving, it may be difficult to relate these results to other laboratory investigations.
Considerable research on using lithium compounds to control ASR was performed under SHRP, with the relevant findings reported by Stark (1992) and Stark, et al. (1993). Because of the large scope of the SHRP study, only selected aspects of the study are discussed here. Stark and his coworkers produced and tested mortar bars according to ASTM C 227, using LiF and Li2CO3 and confirmed the efficacy of lithium in suppressing expansion, provided that a high enough dosage was used. Table 7 summarizes the results of these tests, which used a highly reactive natural aggregate (rhyolite). Expansion was essentially controlled at molar ratios (lithium to sodium plus potassium) of 0.6 for LiF and 0.92 for Li2CO3.
When testing the same rhyolitic aggregate as above and a less reactive granite gneiss, Stark, et al. (1993) reported that molar ratios in the range of 0.75 to 1 were needed to suppress expansion adequately when using ASTM C 1260 (and adding LiOH to the 1N NaOH soak solution). Stark also recognized a pessimum effect, in which an insufficient dosage of lithium actually increased expansion (compared to a control). This was attributed to an increase in the alkalinity (OH- concentration) of the pore solution, which is triggered by the addition of lithium (especially LiOH). As discussed later in this section, other forms of lithium, such as LiNO3, do not tend to raise the pH of the pore solution in concrete, thereby eliminating the pessimum effect.
Diamond and Ong (1992) reported several interesting findings when using LiOH in a series of mortar-bar tests. They reported that a substantial portion of the lithium (> 40 percent) added during mixing was removed rapidly from solution, presumably absorbed by the hydrating cement, and that this absorption was greater for lithium than for sodium or potassium. When mortar bars were cast and tested according to ASTM C 227, a [Li]/[Na+K] molar ratio of 1.2 was required to suppress expansion for mortar containing cristobalite as the reactive aggregate, but this dosage was not quite sufficient to suppress the expansion of similar bars containing beltane opal as the reactive aggregate. The amount of lithium required to suppress ASR-induced expansion was higher for this study than for other published studies, although the reasons for this difference are not clear. Diamond and Ong also confirmed the pessimum effect that was observed by Stark (1992), in which low and moderate amounts of LiOH actually increased expansion, compared to a control mortar without lithium.
| Lithium Compound | Dosage of Lithium Compound (by mass of cement, %) | Molar Ratio [Li]/[Na+K] | Expansion After 1 Year (%) | Relative Expansion Compared to Control (%) | Expansion After 3 Years (%) | Relative Expansion Compared to Control (%) |
|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0.62 | 100 | 0.63 | 100 |
| LiF | 0.25 | 0.3 | 0.59 | 95 | 0.71 | 112 |
| 0.50 | 0.6 | 0.06 | 10 | 0.06 | 10 | |
| 1.00 | 1.2 | 0.02 | 3 | 0.02 | 3 | |
| Li2CO3 | 0.25 | 0.23 | 0.61 | 98 | 0.63 | 100 |
| 0.50 | 0.46 | 0.50 | 81 | 0.58 | 92 | |
| 1.00 | 0.92 | 0.04 | 6 | 0.05 | 10 |
In one of the few studies using LiNO2, Qinghan, et al. (1995) tested mortar bars containing reactive andesite aggregate. The mortar bars were cured for 1 day at 20 °C, then placed in an autoclave under a pressure of 0.28 MPa for 4 hours. After they were removed from the autoclave, the bars were placed in a curing container at 20 °C for four months, then at elevated temperature (40 °C) for long-term measurements. A [Li]/[Na+K] molar ratio of 0.8 was found to reduce expansion significantly for mortars with very high alkali contents (2 percent by mass of cement), but more lithium (based on molar ratio) was needed for mortars with lower alkali contents.
Lumley (1997) used the CPT (ASTM C 1293) to assess the efficiency of LiOH·H2O, LiF, and Li2CO3 in reducing expansion due to ASR. A calcined flint cristobalite was used as the reactive aggregate, and researched a range of lithium dosages. The findings were in broad agreement with most published literature, suggesting that a ratio of equivalent Li2O to equivalent Na2O of 0.33 to 1 (by mass) or a [Li]/[Na+K] molar ratio of 0.62 was sufficient to inhibit expansion.
An important trend in recent years has been the emergence of LiNO3 as the preferred lithium compound in controlling ASR. Stokes, et al. (1997) reported that a major advantage of LiNO3 over other lithium compounds is that LiNO3 does not increase the pH of the pore solution, thereby eliminating the risk of the pessimum effect as described earlier. Using LiNO3 avoids this effect because its addition to cement paste results in an increase in the lithium and nitrate ion concentrations of the pore solution with no significant increase in the hydroxyl ion concentration (Stokes, et al., 1997). The implication of this behavior was confirmed in this study in that all mortar bars containing LiNO3, regardless of dosage, expanded less than the control, which was not the case for previous studies using other lithium compounds (e.g., LiOH). Another important advantage of using LiNO3 as an admixture is that it is closer to a neutral pH than other lithium compounds, making it safer to handle.
Bérubé, et al. (2004) evaluated the influence of LiNO3 or a Li-bearing glass on the chemistry of the pore solution in low- and high-alkali cement pastes stored in sealed containers at different temperatures. For the mixtures incorporating the Li glass, the [Li+] concentration was found to increase with time, temperature, glass fineness, and the [Na++K+] concentration in the pore solution. However, lithium was found to be released at a slow rate while the pH of the pore solution increased slightly, which could explain the limited effectiveness of the lithium glass tested in controlling ASR expansion (Tremblay, et al., 2004). The use of LiNO3 resulted in a slight decrease in pH of the pore solution. It was also found that Li in LiNO3-bearing systems was incorporated into the cement hydrates in greater proportions than Na and K, with Na being always incorporated in greater proportions than K, even in the absence of Li. For all mixtures incorporating LiNO3, the [Li]/[Na+K] molar ratio in the pore solution was usually between 0.30 and 0.45 (i.e., corresponding to only about half of the original amount (0.74) introduced in the system). The authors reported that the overall chemistry of the pore solution and the cement hydrates appeared to not be significantly influenced by temperature, at least in the investigated range of 23 to 60 °C.
A comprehensive study was initiated at the Building Research Establishment (BRE) in 1994 on using lithium compounds (LiOH and LiNO3) to control ASR. Blackwell, et al. (1997) reported on the preliminary findings and Thomas, et al. (2000) gave a more recent update on the status of the project, which includes over 150 concrete mixtures, laboratory testing (using ASTM C 1260 and ASTM C 1293), and exposure block testing at an outdoor site located at BRE in the UK. The program involved the use of several reactive UK aggregates and also included the use of fly ash and slag. Figure 12 summarizes the 3-year expansion data for concrete prisms for the most reactive aggregate, plotted in a similar format to allow comparison with McCoy and Caldwell's findings (Figure 11). A lithium to alkali molar ratio of approximately 0.70 was sufficient to control expansion when using LiNO3, and a higher dosage, around 0.85 (molar ratio) was required for LiOH, mainly due to the impact of LiOH on pore solution pH (as previously discussed). The study also illustrated that the efficacy of lithium in reducing expansion is a strong function of aggregate type and that using low-calcium fly ash in conjunction with lithium increased reductions.
Figure 12. Relative Expansion of Concrete Prisms Containing Lithium Compounds.
Diamond (1999) provided further discussion of the work previously described by Stokes, et al. (1997), including additional insight into the role of LiNO3 in suppressing ASR-induced expansion. He noted that LiNO3 does not raise pore solution pH and demonstrated that the Li+ ions in the pore solution were balanced mainly by NO3- ions and to a smaller extent by SO4-2 ions. Diamond also illustrated that LiNO3 tends to be removed from solution by hydration products, as do other lithium compounds, thus reducing the lithium available to suppress ASR expansion.
Collins, et al. (2004) studied the effect of three lithium additives (LiNO3, LiCl, and LiOH) on the expansion of mortar bars made in accordance with ASTM C 227. While it was shown that all lithium additives were effective in reducing expansion below acceptable limits, different ratios of [Li]/[Na+K] were reported for each additive (i.e., approximately 0.6 for LiOH, 0.8 for LiNO3, and 0.9 for LiCl).
Durand (2000) presented the results of extensive testing using LiOH·H2O, LiF, Li2CO3, and LiNO3 in concrete prisms following ASTM C 1293. Three different reactive aggregates from Canada (from Sudbury, Potsdam, and Sherbrooke) were used in conjunction with different dosages of the four lithium compounds. The results indicate that a molar ratio [Li]/[Na+K] of 0.83 was required to suppress expansion (below 0.04 percent at 2 years) for LiOH·H2O, LiF, and Li2CO3 when using the Sudbury aggregate. A molar ratio of 0.72 was found to be sufficient when using LiNO3 with the Sudbury aggregate. None of the lithium compounds, even when used at their highest dosages (1.66 molar ratio for LiOH·H2O, LiF, Li2CO3 and 0.72 for LiNO3), were able to reduce sufficiently the expansion of concrete containing the Sherbrooke aggregate (a metamorphic schist). The only lithium compounds (and dosages) that adequately controlled expansion of concrete containing Potsdam sandstone were LiOH·H2O and LiF, at molar ratios of 1.66. These findings confirm that the amount of lithium required to suppress expansion depends strongly on aggregate reactivity and also on the specific lithium compound used.
Thomas, et al. (2001) provided preliminary findings from a comprehensive study focusing on the combined use of lithium and fly ash to control ASR. Testing was performed using ASTM C 227 and ASTM C 441, using a highly reactive aggregate from New Mexico, and Pyrex glass. In addition, a modified version of ASTM C 1260 was used to investigate various combinations of lithium nitrate (30 percent solution), six fly ashes, and three cements. ASTM C 1260 was modified by adding LiNO3 to the 1N NaOH soak solution to achieve the same lithium-alkali ratio as that used in the mortar mixture. However, recent unpublished studies have indicated that the results of this modified test method do not correlate well with the results of concrete expansion testing.
Tremblay, et al. (2004a, b, 2006a) presented the results of an extensive testing program using LiNO3 in concrete prisms following ASTM C 1293. In that study, concrete prisms were made with 12 Canadian moderately to highly reactive aggregates of various petrographic natures at various alkali-to-lithium molar ratios. The authors found that the effectiveness of LiNO3 against ASR was related more to the petrographic nature of the aggregate than to its degree of reactivity. Based on a 0.04 percent concrete prism expansion limit after 2 years at 38 °C and R.H. > 95 percent, the "efficient" molar ratios for LiNO3 ranged from 0.56 to 0.74 for six aggregates, 0.93 to 1.11 for three aggregates, while a molar ratio of 1.11 (i.e., 150 percent of the standard dosage) was even found insufficient for three aggregates (Table 8).
Tremblay, et al. (2006b) evaluated the effectiveness of a modified version of the ASTM C 1260 (using various proportions of LiNO3 in the soak solution, with/without lithium in the bar) for a selection of reactive aggregates of various types and reactivity levels from North America; their results are shown in Table 9, which is briefly outlined below:
The limit of 0.08 percent at 28 days has been suggested by some as being a better indicator of the preventive effect of lithium nitrate against ASR. The results in Table 9 show that the minimum lithium-to-alkali molar ratio required from the CPT results ranges from 0.55 to greater than 1.11. Based on the modified ASTM C 1260 results (as purported in the previous version of the guidelines report), when no lithium is used in the bar, this ratio would vary from 0.23 to greater than 0.66 and from 0.33 to 0.73 using the 0.10 percent at the 14-day or 0.08 percent at the 28-day expansion limits, respectively. When 100 percent of the "standard" lithium dosage is used in the bar, the above ratios would vary from 0.18 to 0.45 and from 0.28 to 0.65 using, once again, 0.10 percent at the 14-day or 0.08 percent at the 28-day expansion limits, respectively.
The results in Table 9 show that for most aggregates tested and no matter which limit is used:
Considering that if one were to use the minimum molar ratio in the soak solution, as given in Table 9, to select the dosage of lithium required to control ASR expansion with the aggregates selected, as has been suggested/used by some organizations, this would result in the use of lithium dosages that are significantly lower than those suggested from the results of the CPT. Figure 14 further shows that there is no correlation between the minimum lithium to alkali molar ratios determined from the modified concrete prism and accelerated mortar bar test series performed so far by the authors. More work is currently in progress to evaluate additional modifications of the ASTM C 1260 for lithium-based admixtures.
Fournier, et al. (2003) reported the results of a comparative field and laboratory investigation on the use of SCM and lithium-based admixtures in controlling expansion due to ASR. Concrete mixtures incorporating three highly reactive aggregates of a different petrographic nature were made with LiOH and LiNO3 at molar ratios ranging from 0.74 to 1.11. With the highly reactive NM (rhyolite) and Con (greywacke) aggregates, the use of a molar ratio of 0.74 for LiOH resulted only in a slight reduction in concrete prism and block expansions compared to the control; however, test specimens at a 1.11 molar ratio performed well in the laboratory and after 6 years of field exposure. For the highly reactive NM (rhyolite) aggregate, the use of LiNO3 at molar ratios of 0.74 or 0.93 resulted in similar concrete prism expansions of about 0.031 percent at 2 years (mixtures without added alkalis); however, cracking was observed after 6 years of field exposure for the block with the 0.74 molar ratio. In the case of the highly reactive Sp (siliceous limestone) aggregate, a molar ratio of 0.93 for LiNO3 suppressed concrete prism expansion and offered good performance after 6 years of field exposure.
Berra, et al. (1999) compared the effectiveness of lithium carbonate and lithium nitrate in concrete mixes (CSA test method) made with two natural siliceous aggregates. Also, the ultra-accelerated expansion test in alkaline solution at 150 °C was performed on concrete prisms. The results showed that lithium at the standard molar ratio of 0.74, combined with an alkali loading above 4.5 kg/m3, was ineffective at reducing expansion in the CPT at 2 years below 0.04 percent for the more highly reactive siliceous aggregate in the study. For the second aggregate of lower reactivity, the dosage of lithium was greatly overestimated. While only two aggregate types were studied, the results confirmed that the efficacy of lithium was highly aggregate dependant. This study also stressed that as the alkali content of the concrete increases, the effective molar ratio of lithium to sodium plus potassium also increases significantly. Lithium carbonate was shown to be much less effective than lithium nitrate with the more highly reactive aggregate and was therefore not recommended as a form of lithium for mitigation. In cases where the alkali contents were above 4.5 kg/m3, lithium carbonate was ineffective and, in some cases, especially when the lithium to alkali molar ratio was increased to above 0.74 and the alkali content was also above 4.5 kg/m3, expansion in these prisms exceeded the expansion in the controls.
Perhaps the most promising information from this work was the use of an ultra-accelerated test that was outlined in a previous publication by the same authors (Berra, et al., 1999). In this test, concrete prisms are cast according to the CSA standard with varying molar ratios of [Li]/[Na+K]. After 1 day of curing, they are placed in cylindrical stainless steel containers and immersed in an alkaline solution mimicking that of the pore solution. In mixes using lithium, the soaking solution was also adjusted to yield the same initial alkali to lithium molar ratio as that of the concrete mixture. They showed a strong correlation between the effective dosages of lithium nitrate in this test at 21 days and the effective dosage of lithium nitrate predicted in the CPT at 1 and 2 years for both aggregates. While this represents a promising approach for a possible accelerated version of the test, a larger database will need to be developed that incorporates many types of aggregates before a conclusion as to its validity may be reached.
Mangialardi (2002) used a modified version of the ASTM C 1260 test for assessing the efficacy of lithium nitrate where lithium nitrate was added to both mortar specimens and the soaking solution. While traditional expansion results yielded varying predictions for the proper lithium dosage, the application of the kinetic model proposed by Johnston and Fournier (2000) removed these discrepancies. They also pointed to the fact that although this method is promising, it needs to be applied to a greater variety of ASR-susceptible aggregates before it is relied upon without reservation.
McKeen, et al. (2000)studied the effect of fly ash and fly ash/lithium nitrate combinations on the expansion of mortar bars (the AASHTO T303-96 test, which is virtually identical to ASTM C 1260) for five aggregates from New Mexico. Lithium nitrate was added at 75, 100, and 125 percent of the standard dosage (0.74 [Li]/[Na+K]) in the bar, while no lithium was added to the soaking solution. The researchers measured the lithium concentration in the soaking solution after the bars had been immersed for 24 hours in water (prior to placement in 1N NaOH) and after the 14-day measurement (1N NaOH). They found that, on average, 43 percent of the lithium nitrate had leached out of the bars. They concluded that "some other method should be used to fairly evaluate the effect of LiNO3 on ASR." However, most of the samples (three aggregates with three lithium and three fly ash dosages) exhibited expansions of less than 0.1 percent at 14 days, and these combinations would be said to have passed the test. As a result, a recommendation of a minimum of 25 percent class F fly ash or lithium nitrate at the manufacturer's standard dosage plus class F fly ash at a minimum dosage of 15 percent was made to the New Mexico State Highway and Transportation Department (NMSHTD).
The findings presented by Thomas, et al. (2001) suggest that the beneficial effects of using low-calcium fly ash and lithium together to control ASR are cumulative. Specifically, when either the LiNO3 or low-lime ash were used individually and were unable to suppress expansion completely (e.g., less than 0.10 percent at 14 days in ASTM C 1260), the combined action often was found to be sufficient. The benefits of this approach include needing less lithium, thereby reducing cost, and less fly ash, thereby increasing the early strength development. The combination of lithium and high lime ashes (i.e., > 25 percent CaO) was not found to be synergistic.
Qian, et al. (2002) studied the expansion behavior of dolomite-bearing aggregates in LiOH and KOH solutions. The authors found that the use of LiOH can induce expansion due to alkali-carbonate reaction (ACR) but suppress that due to alkali-silica reaction (ASR), thus offering a mean of differentiating ASR from ACR contributions in the deleterious reaction/expansion of such aggregates.
Table 8 summarizes some of the information provided in this section on various laboratory studies using lithium compounds to suppress ASR expansion. The table particularly focuses on comparisons between the dosage of lithium needed to suppress expansion and does not include the combined use of SCMs with lithium. The findings, as summarized in Table 8, will be used in Chapter 5 to develop specific guidelines for using lithium in new concrete.
Other forms of lithium than those described in this section have also been investigated. For example, Thomas and Stokes (1999) investigated the potential use of decrepitated spodumene (a lithium-containing ore) as a preventive measure and reported that the material was effective when used in sufficient proportions. Cement kiln trials have been also been conducted using spodumene as part of the raw feed, thus producing a clinkerrich in lithium (Stokes, et al., 2000a). A technology has been developed for producing a lithium-containing glass, which can be used as a concrete admixture or interground with portland cement, to suppress expansion due to ASR (Baxter, 2000). This approach of using lithium-bearing glass has been reported as a means of minimizing the uptake of lithium by hydration products, thereby resulting in more efficient use of the active lithium compound in controlling ASR-induced expansion.
Johnston, et al. (2003) has described a new approach for the interpretation of the data from ASTM C 1260 based on a modification of the kinetic model proposed by Kolmogorov-Avrami-Mehl-Johnston. The method has shown some promise in recognizing the potential alkali-reactivity of certain "challenging" aggregates and determining preventive mixtures incorporating supplementary cementing materials (Johnston, et al., 2003); however, the method has yet to be applied to the evaluation of lithium-based admixtures to control ASR.
The findings summarized in this section have dealt exclusively with the effects of lithium compounds on expansion due to ASR. However, it is very important that there are no undesired side effects from any admixture added to concrete that impact other fresh or hardened concrete properties. Fortunately, it has been well-documented that lithium compounds, used in typical dosages to suppress ASR expansion, do not significantly affect other important concrete properties. Most of the more recent investigations have dealt specifically with LiNO3, as it is the most common lithium compound being used today. Studies have shown that fresh concrete properties, such as air content, slump and setting time, and hardened concrete properties, such as strength and permeability, are not altered significantly by the use of LiNO3, and that LiNO3 is compatible with other chemical admixtures (Wang, et al., 1994; Wang and Stokes, 1996; McKeen, et al., 2000; Thomas, et al., 2002).
| Study | Test Method | Reactive Aggregate | Lithium Compound(s) | Minimum Molar Ratio [Li]/[Na+K] Needed to Suppress Expansion* |
|---|---|---|---|---|
| McCoy and Caldwell (1951) | ASTM C 227 | Pyrex glass | LiCl, Li2CO3, LiF, Li2SiO3, LiNO3, Li2SO4 | 0.74 |
| Sakaguchi, et al. (1989) | ASTM C 227 | Pyrex glass, pyroxene andesite sand | LiOH·H2O, LiNO2, Li2CO3 | 0.90 |
| Ohama, et al. (1989) | Autoclave test | Opaline amorphous silica | LiOH·H2O, LiF, Li2CO3 | 0.5% (by mass of cement) for LiF 0.7% (by mass of cement) for LiOH·H2O |
| Stark (1992); Stark, et al. (1993) | ASTM C 227 ASTM C 1260 |
Rhyolite Granite gneiss |
LiOH·H2O, LiF, Li2CO3 | 0.6 (LiF) 0.92 (Li2CO3) 0.75-1.00 (LiOH) |
| Diamond and Ong (1992) | ASTM C 227 | Cristobalite Beltane opal |
LiOH | 1.2 (for cristobalite, more for opal) |
| Qinghan, et al. (1995) | Autoclave | Andesite | LiNO2 | 0.8 (for high-alkali mortars only) |
| Lumley (1997) | ASTM C 1293 | Cristobalite | LiOH·H2O, LiF, Li2CO3 | 0.62 |
| Blackwell, et al. (1997); Thomas (2000) |
ASTM C 1293 | Various UK aggregates | LiOH, LiNO3 | 0.70 (for LiNO3) 0.85 (for LiOH) |
| Durand (2000) | ASTM C 1293 | Canadian aggregates (Sudbury - sandstone, quartzwacke; Potsdam - siliceous sandstone , and Sherbrooke - chloritic schist) | LiOH·H2O, LiF, and Li2CO3, LiNO3 | 0.72 (for LiNO3 with Sudbury) 0.82 (for LiOH·H2O, LiF, and LiCO3 with Sudbury) |
| Collins, et al. 2004 | ASTM C 227 | Crushed, graded borosilicate glass | LiOH, LiNO3, LiCl | 0.6 (LiOH), 0.8 (LiNO3), 0.9 (LiCl) |
| Fournier, et al. 2004 | ASTM C 1293 | Rhyolite (NM) Greywacke (Con) Siliceous limestone (Sp) |
LiOH (NM, Con); LiNO3 (NM, Sp) | LiOH: 1.11 with NM; ~1.0 with Con LiNO3: ~ 0.74 with NM; 0.93 with Sp |
| Tremblay, et al. (2004a, b) | ASTM C 1293 | Canadian aggregates (greywacke-argilite, dolostone, polygenic gravels, rhyolite, siliceous limestones, granite-gneiss) | LiNO3 | Agg. type (1-year CPT exp. %): Molar ratio **
|
* Molar ratios are used unless otherwise noted.
** The information given includes the aggregate (rock) type, the concrete prism expansion at 1 year for the control ASTM C 1293 concrete (data in brackets), and the minimum molar ratio needed to suppress expansion (i.e., to reduce concrete prism expansion to < 0.04 percent at 2 years according to Standard Practice CSA A23.2-28A).
| Aggregate Types | Expansion for Control Specimens | Minimum [Li]/[Na+K] Required to Control ASR Expansion | |||||
|---|---|---|---|---|---|---|---|
| Modified ASTM C 12931 | 0% Lithium in the Bar 2 | 100% Lithium in the Bar 3 | |||||
| ASTM C 1293 Exp. (%) @ 1 year | ASTM C 1260 Exp. (%) @ 14 days | Limit 0.10% @ 14 days | Limit 0.08% @ 28 days | Limit 0.10% @ 14 days | Limit 0.08% @ 28 days | ||
| Granite/gneiss | 0.029 | 0.197 | 0.56 | 0.23 | 0.33 | 0.18 | 0.28 |
| Chloritic schist | 0.082 | 0.138 | > 0.94 | 0.24 | 0.45 | 0.25 | 0.42 |
| Greywacke/argilite | 0.087 | 0.295 | 0.71 | 0.35 | 0.55 | 0.31 | 0.52 |
| Gravel (mixed) | 0.103 | 0.232 | 0.97 | 0.48 | 0.66 | 0.40 | 0.55 |
| Gravel (mixed) | 0.113 | 0.224 | 0.91 | 0.49 | 0.73 | 0.45 | 0.65 |
| Gravel (mixed) | 0.122 | 0.238 | 0.66 | 0.30 | 0.43 | 0.21 | 0.30 |
| Gravel (mixed) | 0.131 | 0.375 | 0.58 | 0.48 | 0.56 | 0.27 | 0.41 |
| Greywacke | 0.142 | 0.273 | > 1.11 | 0.41 | 0.68 | 0.39 | 0.61 |
| Rhyolite | 0.151 | 0.414 | 0.63 | 0.25 | 0.45 | 0.21 | NA |
| Siliceous limestone | 0.162 | 0.305 | 1.04 | 0.52 | 0.65 | 0.42 | NA |
| Siliceous limestone | 0.199 | 0.246 | > 1.11 | 0.32 | 0.52 | 0.25 | 0.40 |
| Gravel (volcanic) | 0.212 4 | 0.966 | 0.74 | >>0.60 | >>0.60 | 0.45 | 0.59 |
| Sand (cherty) | 0.590 5 | 0.656 | 0.55 | 0.63 | 0.70 | 0.36 | 0.47 |
1 Minimum molar ratio ([Li]/[Na+K]) required to meet the 2-year 0.04 percent concrete prism expansion limit (as per CSA Standard Practice A23.2-28A).
2 Minimum molar ratio ([Li]/[Na+K]) required in the 1N NaOH soaking solution to meet either the 14-day 0.10 percent or 28-day 0.08 percent accelerated mortar bar expansion limits, as per modified ASTM C 1260 (0 percent lithium in the bar).
3 Minimum molar ratio ([Li]/[Na+K]) required in the 1N NaOH soaking solution to meet either the 14-day 0.10 percent or 28-day 0.08 percent accelerated mortar bar expansion limits, as per modified ASTM C 1260 (100 percent of the standard dosage of lithium in the bar).
4 From Fournier, et al. (2003).
5 From Folliard and Ideker (2005).
Figure 13. Comparison Between the Minimum Lithium to Alkali Molar Ratios to Control ASR Expansion Based on CPT and AMBT Results (Plot of Data in Table 9) (Tremblay, et al. 2005).
In comparison to the number of studies on using lithium as an admixture in new concrete, there have been very few laboratory-based studies on post-treating hardened concrete to arrest expansion due to ASR. There have been several field applications, as described in the next chapter, but the following briefly summarizes laboratory research on the topic.
Sakaguchi, et al. (1989) allowed both high-alkali mortar bars (containing Pyrex glass) and concrete prisms (containing a reactive aggregate) to expand considerably (0.2 percent expansion for mortar, 0.1 percent for concrete), and then soaked the specimens in LiNO2 or LiOH·H2O solution. For both mortar and concrete, future expansion was essentially significantly retarded or, in some cases, prevented.
Stark, et al. (1993) confirmed that treating hardened mortar, previously subjected to ASR-induced expansion, by soaking it in LiOH solution was an effective method of suppressing further expansion. They noted that a key issue in field applications would be to ensure adequate LiOH penetration.
Recently, Stokes, et al. (2000b) reported on the development of a material for controlling future expansion of hardened concrete. The material is a lithium nitrate-based solution, but also contains a proprietary blend of surfactants to aid in penetrating hardened concrete. It was reported to be 50 percent more effective than LiNO3, by itself, or three times more effective than LiOH, by itself. Stokes, et al. (2000b) also noted that the specific time of treating hardened concrete plays a major role in the subsequent efficiency of lithium compounds in controlling expansion, presumably because of increased permeability and damage of ASR-affected concrete.
There is relatively little guidance provided in specifications related to the use of lithium in concrete. Current specifications within ASTM and CSA do not include guidance on using lithium compounds, although the next version of CSA specifications is expected to provide information on using lithium in new concrete. AASHTO (2000) has provided guidance on using lithium compounds in new concrete as part of the guide specifications developed by the Lead States Program. Several State highway agencies in the United States allow for the use of lithium in their specifications, including Delaware, Idaho, Maine, New Mexico, South Dakota, Texas, Washington State, and Wyoming.
The BRE (2002) has published guidelines recently for using lithium admixtures in concrete, as shown in Table 10. The BRE recommendations take into account aggregate reactivity, fly ash dosage (if used), type of lithium compound, and total alkali content of the concrete mixture. Table 10 is only applicable for concrete with a total alkali content of less than or equal to 5 kg/m3. The classification of aggregates (high reactivity or normal reactivity) generally is based on mineralogy, as opposed to performance tests, but a test similar to ASTM C 1293 may be used if aggregate reactivity is in question. Guidance is not provided by BRE on using slag in conjunction with lithium compounds. When fly ash is used, the alkali contribution of the fly ash in calculating the total mixture alkali content is as follows:
| Aggregate Type | Lithium Compound | Fly Ash, % (by mass of cementitious materials) | Lithium Dosage | ||
|---|---|---|---|---|---|
| Mass Addition (kg per kg of Na2Oe) | Volume Addition (L of solution admixture per kg of Na2Oe) | Molar Ratio [Li]/[Na+K] | |||
| High Reactivity | LiOH·H2O (solid) | 0-14 | 1.30 | - | 0.96 |
| 15-25 | 1.00 | - | 0.74 | ||
| LiNO3 (30% solution) | 0-14 | 5.95 | 5.00 | 0.80 | |
| 15-25 | 5.20 | 4.40 | 0.71 | ||
| Moderate Reactivity | LiOH·H2O (solid) | 0-25 | 0.75 | - | 0.56 |
| LiNO3 (30% solution) | 0-25 | 3.75 | 3.15 | 0.51 | |
For convenient comparison to data presented earlier in this chapter, the last column of Table 10 shows the molar ratio for each of the standard lithium dosages. For the same aggregate type and fly ash content, higher dosages of lithium (based on molar ratio) are recommended for LiOH·H2O than for LiNO3, which recognizes the superior performance of LiNO3 in controlling expansion due to ASR.
This chapter has reviewed a wide range of research performed using lithium to combat ASR-induced expansion. The following are some of the key findings: