CHAPTER 4. RESULTS AND DISCUSSION #1
TASK 1.1. STRESS CORROSION CRACKING
Initial pH of the ambient temperature test solution was 13.45; however, the pH decreased to
13.30 with incremental Cl- additions due to the common ion effect. No strain changes that could be related to crack development were noted, and visual low power microscopic inspection
failed to reveal any cracking. This was the case for both the ambient and elevated temperature
exposures. It is concluded that the specimens were not susceptible to stress corrosion cracking in
the simulated pore solution.
TASK 1.2. CORROSION PROPERTIES OF TYPE 2304 SS REINFORCEMENT
Accelerated Test Method
Figure 11 plots current density versus exposure time for the 10 identical 2304 SS specimens.
The pH data is also shown, which appears as a near horizontal line slightly above a value of
12 and [Cl-] versus time according to the incremental additions. Current density was between
10 and 12 μ A/cm2 initially but decreased to zero to 2 μA/cm2 during the first few days of
exposure, presumably reflecting repair of defects in the passive film. For most specimens, a
definitive transition from this low current density to much higher values occurred at a particular
time, which reflected the onset of active pitting. The time at which this occurred covered a
relatively broad range from 295 hours for specimen 9 to 1,608 hours for specimen 7. The data
interruption near 1,000 hours resulted from a power outage that lasted several days due to
Hurricane Katrina in August 2005.
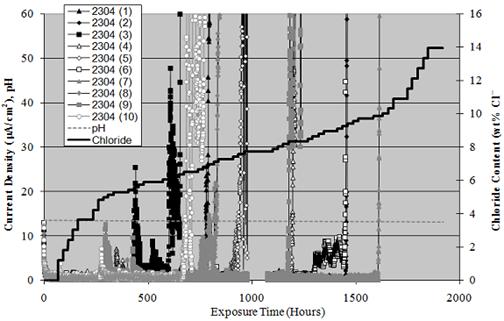
Figure 11. Graph. Accelerated corrosion test data.
Table 4 shows the [Cl-] at which individual specimens activated. As such, these values are
indicative of the critical chloride concentration for initiation of corrosion, CT. The fact that these
values extend over a range suggests that the threshold concentration is a distributed parameter
rather than a discrete number as reported previously by others.(16-18) Figure 12 shows a
cumulative distribution function plot of these data which allows projection of the probability of
corrosion initiation at a particular [Cl-]. This analysis assumes the data are normally distributed.
By way of comparison, a companion study using this experimental method reported CT for black
bar as 0.24–0.30 wt percent Cl-(19). Thus, CT for 2304 SS was about 17 times greater than for
black bar according to this experimental method. However, the companion study referenced
above questioned accuracy of this approach for correctly ranking reinforcements according to CT
compared to performance in concrete.(19)
Table 4. Chloride concentrations at activation in accelerated tests.
| Specimen Number |
[Cl-] wt Percent |
| 1 |
6.83 |
| 2 |
9.25 |
| 3 |
5.61 |
| 4 |
8.34 |
| 5 |
6.52 |
| 6 |
9.25 |
| 7 |
9.86 |
| 8 |
6.83 |
| 9 |
5.00 |
| 10 |
7.58 |
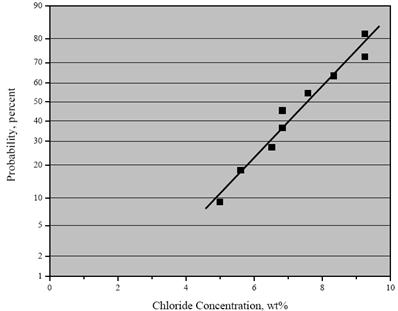
Figure 12. Graph. Cumulative distribution plot of CT for 2304 SS from accelerated testing.
Concrete Specimen Exposures
The SDS specimens were exposed for 929 days. Figure 13 shows the resultant potential data. For
the most part, potentials were in the range -150 to -200 mVSCE; however, specimen 2304-1 in
particular had negative excursions which reached -356 mVSCE on one occasion. Figure 14 shows
the corresponding macrocell current data. With the exception of one reading for specimen
2304-1, the currents were below the detection limit and considered zero. The one finite current
reading of 0.1 μ A for specimen 2304-1 occurred at the same time as the most negative potential
excursion for this same specimen (404 days exposure) that was mentioned previously.
Apparently, the specimen exhibited momentary corrosion activity followed by repassivation.
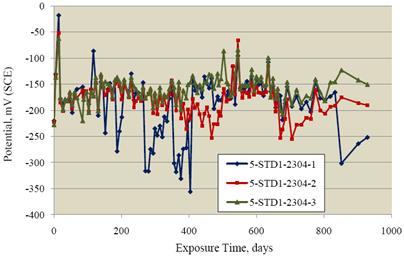
Figure 13. Graph. Potential data for the 2304 SS-reinforced concrete specimens.
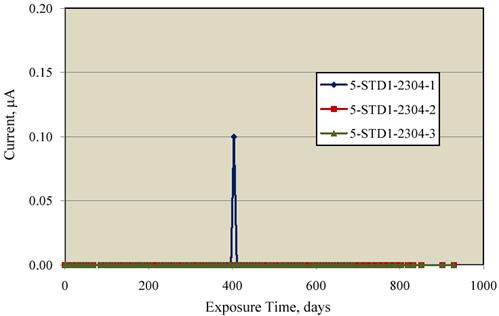
Figure 14. Graph. Macrocell current data for the 2304 SS-reinforced concrete specimens.
While no cores were taken from the 2304 SS-reinforced SDS specimens, 75-mm-diameter cores
were taken from identical companion specimens subjected concurrently to the same exposure.
However, the cores were taken at different times. Once acquired, the cores were dry sliced
parallel to the top surface at 6.4-mm intervals, and the individual slices were ground to powder.
The powder samples were then analyzed for [Cl-] using the FDOT wet chemistry method.(20) Figure 15 shows a plot of [Cl-] data for these as a function of depth into the concrete.
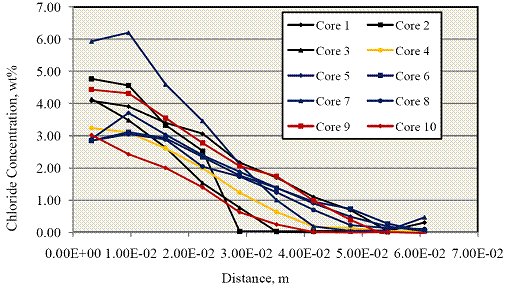
Figure 15. Graph. Concrete chloride concentration profiles determined from 10 cores.
From each of the above [Cl-] profiles, a value for the effective diffusion coefficient, De, was
calculated using a least squares fit to the one-dimensional solution to Fick's second law as seen
in the following equation:
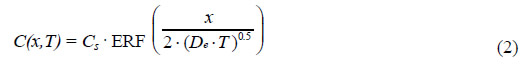
Where:
| C(x,T) |
= [Cl-] at depth, x, into the concrete after time, T. |
| Cs |
= [Cl-] at the concrete surface. |
| ERF |
= Gaussian error function. |
| D |
= Effective diffusion coefficient. |
This solution assumes that Cs and De are spatially and chronologically constant, whereas they
are, in fact, distributed parameters and may vary with exposure time and concrete age.(21) The
solution also assumes that initial [Cl-] in the concrete was zero. Using the average De for the 10
determinations (2.59·10-11 m2/s), [Cl-] was calculated at the top bar depth at 929 days using
equation 1 and assuming Cs = 18 kg/m3(7.22 wt percent cement basis). This yielded a value of
12.5 kg/m3(4.51 wt percent cement). It is concluded, assuming the momentary potential and
macrocell current activity cited above in conjunction with figure 13 and figure 14 did not
constitute corrosion initiation, that CT for 2304 SS exceeds this value. In the recently completed companion study references above, CT for black bar at a probability of 2-percent activation was
1.0 kg/m3(0.35 wt percent cement) and for 20-percent activation 1.9 kg/m3(0.69 wt percent
cement).(19) If the above cited minimum CT for 2304 SS (12.5 kg/m3) were to correspond to
2-percent probability of corrosion initiation for this reinforcement, then CT for 2304 SS exceeds
that of black bar by a factor of 12.5. If, on the other hand, this CT for 2304 SS pertains to
20-percent probability of activation, then the improvement relative to black bar is by a factor
greater than 6.6.
|
