CHAPTER 3. RESEARCH APPROACH #1
TASK 1.1. STRESS CORROSION CRACKING
Procedure
For these experiments, 2304 SS bars approximately 0.50 m long were bent to a radius bend four
times the diameter of the bar (a 4D-radius) as seen in figure 1. Next, a strain gauge was mounted
on the outside diameter of the bent bar approximately 15 cm from the midpoint of the bend.
Specimens were then mounted individually in a vise, bent further to the point where the two side
lengths were parallel, and restrained in this position using a custom configured C-clamp. Care
was exercised to ensure that specimens did not relax when removed from the vise to avoid a
situation where the critically stressed region (outside diameter at the center of the bend) went
into residual compression. Figure 2 shows a schematic representation of a specimen in the bent
and restrained state. The location of the maximum tensile stress, strain gauge, and C-clamp are
shown as blue, red, and green, respectively.

Figure 1. Photo. 2304 SS bar after bending.
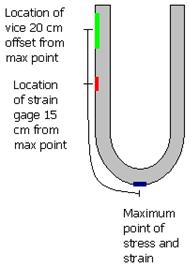
Figure 2. Illustration. A bent specimen in the restrained position.
All exposures were performed in two polyethylene tanks using a simulated pore solution that consisted of 0.30N KOH + 0.05N NaOH (initial pH = 13.44), where N is a chemical concentration unit indicating normal. One tank was maintained at room temperature, and chlorides were added to this in 1-2 weight (wt) percent increments on alternate days. The solution was titrated for OH- before, and after, Cl- was added to determine any pH change with time. Figure 3 shows one of the test tanks, and the two ends of a specimen can be seen protruding through the cover. Lead wires from the strain gages appear in the foreground of the figure. Likewise, figure 4 shows a top view of two specimens positioned in a tank with the cover removed. The second tank was maintained at 65 °C and a constant chloride content of 15 wt percent. Each specimen was placed in a separate plastic cylinder that was wrapped with insulation. Temperature was maintained by a heating element and a temperature probe in the plastic cylinder, the latter being connected to a thermostat.

Figure 3. Photo. Test tank with cover.
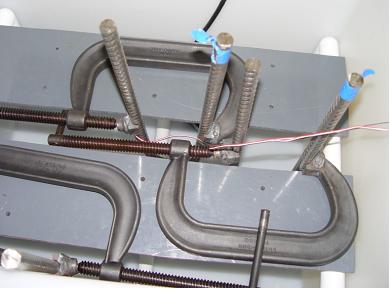
Figure 4. Photo.Top view of two specimens with C-clamps in the test tank.
Figure 5 shows two specimens positioned in temperature-controlled cylinders. In all cases, the bend was submerged in the simulated pore solution to a depth of 5 cm. The gauges were monitored for any strain decrease that would be indicative of cracking. Specimens were also examined daily. The higher temperature exposures were terminated after about 1 month, but the ambient temperature exposures continued for 1 year.

Figure 5. Photo. High temperature experiment arrangement.
TASK 1.2. CORROSION PROPERTIES OF TYPE 2304 SS REINFORCEMENT
Accelerated Corrosion Test Procedure
The accelerated test method for these experiments consisted of potentiostatic polarization of
10 identical 152-mm-long 2304 SS reinforcing bar specimens at +100 mVSCE using a single
locally designed and constructed potentiostat, where SCE is the potential versus saturated
calomel electrode. A 10-Ω resistor was in series with each specimen, and voltage drop was
monitored. Exposure was in synthetic pore solution of the same composition noted above
(0.30N KOH + 0.05N NaOH) to which chlorides were incrementally added. This potential
(+100 mSCE) is considered conservative in that it exceeds the free corrosion potential that
should occur in actual structures. In the absence of or with low chlorides, even black steel should
be passive at this potential such that polarization should occur readily with low current demand.
After a steady state was achieved after several days in the synthetic pore solution, chlorides were
incrementally added. Corrosion was considered to have initiated once current density increased
to 10 µ A/cm2, as calculated from the voltage drop across the 10-Ω resistor. The Cl- concentration
that resulted from this achieved density was taken as the critical value for pitting and corrosion
initiation, and it served as an important materials selection and design parameter in LCCA.
Exposure of individual specimens was terminated once corrosion was initiated. Figure 6 shows a
specimen ready for exposure with epoxy end mounts and an electrical lead, figure 7 shows a
schematic representation of the experimental setup, and figure 8 shows the test system.

Figure 6. Photo. Straight as-received 2304 SS bar with epoxy-mounted ends
and an electrical lead.
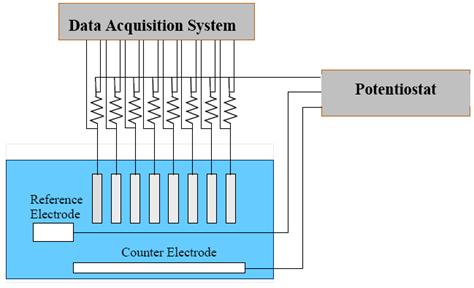
Figure 7. Illustration. Accelerated experimental arrangement.
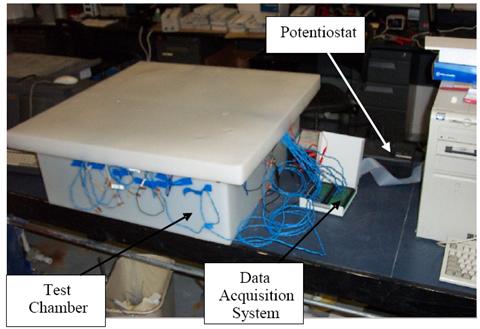
Figure 8. Photo. Test system.
Reinforced Concrete Exposures
Long-term exposure of three concrete slab specimens that were reinforced with 2304 SS was also
performed. The concrete mix, designated STD1, had five bags of cement and a 0.50 w/c which
yielded high permeability. The coarse aggregate was Florida limestone, and the fine aggregate
was a local silica sand. The target mix design is shown in table 3.
Table 3. Concrete mix design.
| Material |
Quantity |
| Cement (bags) |
5 |
| Cement, kg |
213 |
| Water, kg |
107 |
| w/c |
0.50 |
| Fine aggregate, kg |
652 |
| Coarse aggregate, kg |
753 |
The specimens were fabricated at the Florida Department of Transportation State Materials
Office (FDOT-SMO) in Gainesville, FL, and they were designated as simulated deck slabs
(SDS). These were intended to simulate a northern bridge deck or slab exposed to chlorides from
either deicing salts or sea water. Figure 9 provides a schematic illustration of the specimen
design where three straight bars comprised a top layer, and three bars comprised a bottom layer.
Concrete cover for all bars was 25 mm, and triplicate specimens were prepared. Prior to casting,
the reinforcement was degreased by cleaning with hexane, and heat shrink tubing was applied at
the bar ends. This application provided an electrical barrier at the concrete-reinforcement
interface, leaving only the center portion of the reinforcement within approximately 25 mm of
the exposed concrete surface. The casting procedure involved placing freshly mixed concrete in
the specimen molds in two lifts followed by consolidating each lift for 20-30 s on a vibration
table. The first lift filled the specimen mold approximately half full, and the second lift
completely filled the mold. The surface of the specimens was troweled smooth using a wooden
or metal float. After 24 hours, the molds were dissembled. The specimens were removed, placed
in sealed plastic bags, and stored for 6 months.
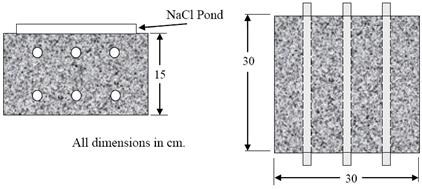
Figure 9. Illustration. Simulated deck slab specimen design.
Upon delivery to Florida Atlantic University (FAU), an electrical connection was established
between bars in each of the two layers of each slab using a SS wire in conjunction with a drilled
hole and connection screw at one end of each bar. Periodically, a 10-Ω resistor was temporarily inserted in the circuit between the two bar layers, and voltage drop across this was measured.
From this procedure, the macrocell current was calculated. The specimen sides were coated with
an ultraviolet-resistant paint and inverted relative to their orientation at casting. A plastic bath with a vented lid was then mounted on what was the bottom formed face. Prior to ponding, the
specimens were stored outdoors in a covered location for 2 months at the FAU Sea Tech
Campus, which is approximately 300 m inland from the Atlantic Ocean southeast of
Ft. Lauderdale, FL. The initial week of ponding was with potable water to promote saturation
or a high humidity pore structure so that upon ponding, diffusion and not sorption would be
the primary Cl- ingress mechanism. This was followed by cyclic 1 week wet/1 week dry
ponding with 15 wt percent sodium chloride (NaCl). The salt water ponding commenced on
August 10, 2005. Figure 10 shows the three specimens under test.

Figure 10. Photo. SDS specimens reinforced with 2304 SS under test.
|
