U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
 |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
Publication Number: FHWA-HRT-06-133 Date: March 2007 |
Alkali-silica reaction (ASR) was first identified as a form of concrete deterioration in the late 1930s (Stanton 1940). About 10 years later, it was discovered that lithium compounds could be used to control expansion due to ASR. Recently there has been increased interest in using lithium technologies both to control ASR in new concrete and to retard the reaction in existing ASR-affected structures.
This book provides information on lithium, its origin and properties, and its applications. The mechanism of alkali-silica reaction is discussed together with methods of testing to identify potential alkali-silica reactive aggregates. Traditional methods for minimizing the risk of damaging ASR are presented; these include the avoidance of reactive aggregates, controlling the levels of alkali in concrete, and using supplementary cementing materials such as fly ash, slag, and silica fume.
The final two sections of the book discuss the use of lithium, first as an admixture for new concrete construction, and second as a treatment for existing concrete structures affected by ASR.
The information in this document was obtained primarily from the FHWA publication Interim Recommendations for the Use of Lithium to Mitigate or Prevent Alkali-Silica Reaction (ASR) (Publication No. FHWA-HRT-06-073). For further information, the reader should refer to the above publication.
Lithium is the third element in the periodic table (see figure 1) and is denoted by the chemical symbol "Li." Its atomic number is 3 and its atomic mass is 6.941 grams (g), making it the third lightest element on earth after hydrogen (H) and helium (He).
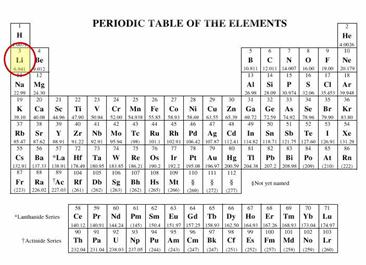
Figure 1. Periodic table showing the position of lithium.
As a pure element, lithium is a soft, silver-white metal and belongs in the Alkali Metal Group together with sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
Lithium has only one electron in its outer shell (i.e., valence = +1) which makes the pure metal very unstable and reactive to moisture. Stable lithium compounds can be manufactured for commercial use; examples are lithium carbonate (Li2CO3), lithium chloride (LiCl), lithium sulfate (Li2SO4), and lithium nitrate (LiNO3).
Lithium metal does not occur naturally in the environment, and lithium is most commonly found in lithium-bearing minerals such as spodumene (LiAlSi2O6) in pegmatite rocks or as dissolved salt such as lithium chloride (LiCl) in brines (see figures 2 and 3). Table 1 provides a list of common lithium-containing minerals.
 |
 |
Figure 2. Photograph of lithium metal. |
Figure 3. Photograph of the lithium- bearing mineral spodumene. |
Table 1. Principal lithium minerals and their sources (after Lumley, 1997).
| Mineral | Formula | Locations of Deposits (in Alphabetical Order) |
|---|---|---|
Spodumene |
LiAlSi2O6 |
Australia, Brazil, Canada, China, Russia, United States |
Petalite |
LiAlSi4O10 |
Australia, Brazil, Namibia, Russia, Sweden, Zimbabwe |
Amblygonite |
(Li,Na)Al(PO4)(F,OH) |
Brazil, Canada, Mozambique, Namibia, Rwanda, South Africa, Suriname, Zimbabwe |
Lepidolite |
K(Li,Al)3(Si,Al)4O10(F,OH)2 |
Brazil, Canada, Namibia, Zimbabwe |
Eucryptite |
LiAlSiO4 |
Zimbabwe |
Spodumene is the most abundant of the lithium-containing minerals from which lithium is extracted. To extract lithium in this process, spodumene ore concentrate is first heated in a rotary kiln at about 1,000 °Celsius (C) (1,832 °Fahrenheit (F)) to decrepitate the spodumene. This clinker is then roasted with sulfuric acid at about 250 °C (482 °F) to leach out an aqueous extract of lithium sulfate. This lithium sulfate is then converted to lithium carbonate, the primary compound from which other lithium compounds are manufactured.
Processing ore deposits is energy-intensive, but less expensive lithium extraction methods exist. For instance, much of the lithium produced today is extracted from subsurface salt brine deposits. The largest deposits of lithium-containing brines are found in Argentina and Chile. Solar evaporation is used to precipitate the salts, which are then processed chemically to separate the lithium (as Li2CO3) from the other compounds; figures 4 and 5 show aerial views of these deposits.
 |
 |
Figure 4. Aerial view of lithium-bearing brines in Argentina (Salar del Hombre Muerto). |
Figure 5. Aerial view of lithium-bearing brines in Chile (Salar de Atacama). |
Lithium carbonate (Li2CO3) is used as a feedstock for other processes to produce a variety of lithium compounds which are then used in a wide range of applications. Table 2 provides a list of common lithium compounds and different applications.
The main application for lithium in the construction industry is in the formulation of chemical admixtures for concrete. Various lithium compounds (Li2CO3, LiOH, Li2SO4) are used in the formulation of set accelerators for calcium-aluminate-cement concrete and both lithium hydroxide monohydrate (LiOH•H2O) and lithium nitrate (LiNO3) have been used to control ASR in portland cement concrete.
Table 2. List of lithium compounds and applications for lithium.
| Forms of Lithium | Common Applications |
|---|---|
Lithium metal (Li) |
Air treatment |