U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| TECHBRIEF |
| This techbrief is an archived publication and may contain dated technical, contact, and link information |
| Publication Number: FHWA-HRT-16-017 Date: October 2015 |
Publication Number: FHWA-HRT-16-017 Date: October 2015 |
To assess the current state of knowledge and practice, the research team reviewed technical and trade literature that involved large-scale ACM use, made site visits to evaluate pavements, and communicated with material suppliers. In addition, the researchers met with producers and users and created a technical working group comprised of user stakeholders who represented various State and Federal agencies.
For the literature review, the research team focused on hydration mechanisms, set times, usage of admixtures, and durability testing. The literature review offered insight into historical use of ACMs throughout the United States and abroad, and enabled the research team to obtain observations from test sections throughout the United States. Table 3 summarizes general information found in the literature about each of the ACM classes investigated. Further information on hydration kinetics research needs is available in a joint National Institute of Standards and Technology–FHWA publication and road map for coordinated cement research. The joint publication was developed following an International Summit on Cement Hydration Kinetics and Modeling.[13]
Table 3. Basic ACM information from literature review.
| LITERATURE REVIEW TOPIC | CALCIUM ALUMINATE CEMENT (CAC) | CALCIUM SULFOALUMINATE CEMENT (CSA) | CALCIUM SULFOALUMINATE BELITE CEMENT (CSAB) | MAGNESIUM PHOSPHATE CEMENT (MPC) | ALKALIACTIVATED (AA) BINDERS/ GEOPOLYMERS | CARBONATE SYSTEMS |
| BASIC HYDRATION | Calcium aluminate
compounds react
with water to
form one of three
calcium aluminate
hydrate phases
(6CaOAl2O3.10H2O, 2CaO.Al2O3.8H2O, or 3CaO. Al2O3.6H2O), depending on temperature and the amount of moisture present. Only the 3CaO. Al2O3.6H2O form is stable long term, with the phase transformation generally referred to as conversion. |
4CaO.3Al2O3.SO3 (Klein's compound) reacts quickly with water to form monosulfate, ettringite or stratlingite. | Klein's compound is used in conjunction with high belite content cement to create a binder system capable of generating high early strengths in addition to continued improvement of properties over time from the slow hydration of the belite. | Magnesia, NH4H2PO4 (ammonium phosphate), and water react to form NH4MgPO4.6H2O. Chemical bonding is formed by a rapid through-solution acid-base reaction between dead burned magnesia and phosphate. [14] | AA binders are formed through three steps: 1. Covalent Si-O-Si and Al-O-Al bonds are broken down by a high pH solution; 2. Products accumulate; and 3. an amorphous aluminosilicate structure reforms and precipitates. | Carbonate "cement" powder and sand are mixed with water and CO2. The cement reacts with CO2 to form calcium carbonate and silicate hydrate gel, gaining strength through solidification. |
| TYPICAL WATER-TO CEMENT RATIOS USED |
0.30–0.40 [15–18] ≤0.4 is necessary for good longterm strength.[19] | 0.20–0.70[7, 20–26] | 0.40–0.70[27–33] | 0.10–0.52[14, 34–38] | 0.28–1.10[39–46] | N/A |
| COMPRESSIVE STRENGTHS (POUNDS PER SQUARE INCH) |
3,000–4,200 @ 3 hrs 6,500–7,900 @ 28 days with 0.38 w/c.[47, 48] |
87–3,800 @ 3 hrs depending on accelerator/ retarder usage.[20, 23] | 1,200 @ 28 days (for 0.8 w/c) 5,000 @ 28 days (for 0.26 w/c).[49, 50] | 2,900–7,300 @ 3 hrs. [37, 51] | 2,000 @ 3 hrs 10,000–20,000 @ 28 days [52] | 9,000 @ 24 hrs (personal communications— Solidia Technologies, 25 Sept 2014). |
| TIME
TO INITIAL SET AT 23°C WITH NO ADMIXTURES |
2–6 hrs.[53] However, no superplasticizers are effective for longer than 15 min, so working time is very limited (personal communications— Kerneos Inc., May 2015). | 8–22 min.[20, 23] | 10–15 min with no admixtures.[28] | 9 min.[37] | As little as 35 min (based on U.S. Army Engineer Research and Development Center in-house report | Product is currently available as a precast product only. Elements typically require 24 hrs to cure. |
| RETARDING ADMIXTURES |
Retarders recommended by CAC manufacturer include lignosulfonates, Melmet 50, Chryso AL810, Grace Daratard 17, and BASF Pozzolate 100XR (personal communications— Kerneos Inc., May 2015). | Organic acids, such as citric acid, tartaric acid in doses not exceeding 0.25% by mass of cement.[48] | Nanoparticles can be used to accelerate hydration; dopants can be used during clinkering to distort belite structure to make it more reactive.[49, 54] | Borax can be used in dosages of 2–25% by weight of cement, but higher dosages may affect compressive strength.[35, 51, 55] | Sodium silicate can increase the speed of binder nucleation and polymerization. [56, 57] | N/A |
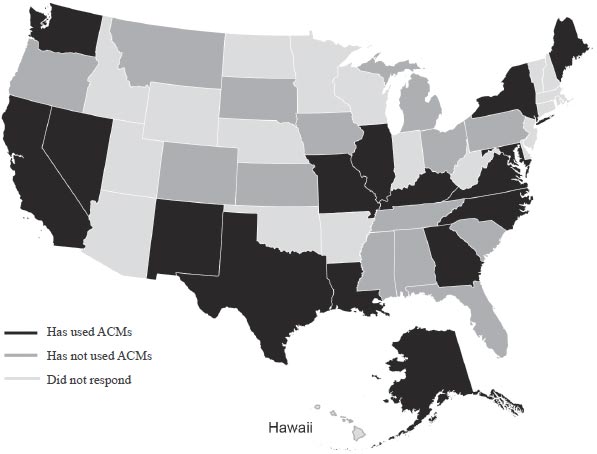
In addition, the research team was able to summarize survey results on the usage of ACMs by State departments of transportation (DOTs). The American Association of State Highway and Transportation Officials’ Subcommittee on Materials conducted a survey about ACMs. Of the 27 responding States, 14 States (50 percent) indicated that they had experience using ACMs, as shown in figure 1. The specific ACMs used by each State and anecdotal assessments of performance by ACM class are provided in table 4.
© The Georgia Institute of Technology
Figure 1. Survey responses for States’ ACM usage.