U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| REPORT |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
| Publication Number: FHWA-HRT-11-060 Date: November 2011 |
Publication Number: FHWA-HRT-11-060 Date: November 2011 |
This study involved the evaluation of 11 systems in which ECR was combined with another corrosion-protection system. The research included seven bar types: one uncoated and six with a fusion-bonded epoxy coating fabricated from the same heat of steel. Uncoated conventional reinforcing steel and conventional ECR served as the controls. The multiple corrosion-protection systems included conventional ECR in conjunction with one of three corrosion inhibitors; bars that were treated with a primer coating containing microencapsulated calcium nitrite (a corrosion inhibitor) prior to coating with conventional epoxy; bars with improved adhesion between the epoxy and the reinforcing steel, obtained through the use of either a zinc chromate pretreatment or special epoxies with higher adhesion; the combination of bars with an improved adhesion epoxy and the addition of calcium nitrite to the mortar or concrete; and bars with multiple coatings consisting of a 50-![]() m (2-mil) layer of 98 percent zinc and 2 percent aluminum that was, in turn, coated with a conventional epoxy. In addition, a second heat of conventional reinforcement was used to evaluate the corrosion performance of uncoated steel cast in concrete containing one of the three corrosion inhibitors used in the study. The systems are listed in table 1 along with the shorthand notation used in this report.
m (2-mil) layer of 98 percent zinc and 2 percent aluminum that was, in turn, coated with a conventional epoxy. In addition, a second heat of conventional reinforcement was used to evaluate the corrosion performance of uncoated steel cast in concrete containing one of the three corrosion inhibitors used in the study. The systems are listed in table 1 along with the shorthand notation used in this report.
System | Abbreviation |
Control | |
Conventional uncoated reinforcing bars | Conv. and Conv.2 |
Conventional ECR | ECR |
Epoxies with increased adhesion | |
Chromate pretreatment | ECR(Chromate) |
DuPont™ coating | ECR(DuPont) |
Valspar® coating | ECR(Valspar) |
Corrosion inhibitors in mortar or concrete | |
Uncoated bars with Ca(NO2)2 | Conv.2(DCI) |
Uncoated bars with Hycrete | Conv.2(HY) |
Uncoated bars with Rheocrete® 222+ | Conv.2(RH) |
ECR with calcium nitrite | ECR(DCI) |
ECR with Hycrete™ | ECR(HY) |
ECR with Rheocrete® 222+ | ECR(RH) |
3M™ primer containing calcium nitrite | ECR(primer/Ca(NO2)2) |
Epoxies with increased adhesion plus calcium nitrite in mortar or concrete | |
Chromate pretreatment | ECR(Chromate)-DCI |
DuPont™ coating | ECR(DuPont)-DCI |
Valspar® coating | ECR(Valspar)-DCI |
Bars with multiple coatings | MC |
Uncoated Conventional Steel (Conv.)
With the exception of the tests to evaluate uncoated bars in concrete containing corrosion inhibitors, all tests of both uncoated and coated bars involved the use of a single heat of No. 16 (No. 5) Grade 420 (60) ASTM A615 reinforcing steel, identified as "Conv."(12) The uncoated reinforcement used in the corrosion inhibitor tests is identified as "Conv.2." Chemical analysis is shown in table 2.
Table 2. Chemical analysis of steel by percent.
| Steel | Bar Size No. | Heat Number | C | Mn | Si | P | S | Cr | Ni | Mo | Cu | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conv. | 16 (5) |
231159 | 0.43 | 0.95 |
0.21 | 0.014 | 0.046 | 0.20 | 0.17 | 0.038 | 0.49 | 0.0005 |
Conv.2 | 16 (5) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
C = Carbon; Mn = Manganese; Si = Silicon; P = Phosphorus; S = Sulfur; Cr = Chromium; Mo = Molybdenum;
Cu = Copper; N = Nitrogen; B = Boron.
N/A indicates chemical analysis not available.
The conventional reinforcement coated with fusion-bonded thermoset epoxy used as the control was coated with Scotchkote™ 413, manufactured by 3M™.
A number of techniques have been used to improve the adhesion of epoxy coatings to steel. Three systems were evaluated in this study. The first involved pretreatment of uncoated steel with zinc chromate prior to the application of the epoxy coating. This procedure is used in Canada for all ECR, but because it involves the use of hexavalent chromate, which presents a significant environmental problem, it is not widely used in the United States. As an alternative, DuPont™ and Valspar® have developed epoxy powders with improved adhesion to reinforcing steel that do not require pretreating the bars. The three systems are identified as ECR(Chromate), ECR(DuPont), and ECR(Valspar).
Three corrosion inhibitors, one inorganic and two organic, were studied. Calcium nitrite is the most widely used inorganic corrosion inhibitor in U.S. practice. Because calcium nitrite acts as a set-accelerator, dci® s, an admixture produced by Grace Construction Products that contains a retarder, was used in this study. The organic corrosion inhibitors were Rheocrete® 222+, a water-based combination of amines and esters produced by BASF Admixtures, and Hycrete™, a salt of alkenyl-substituted succinic acid produced by Broadview Technologies for this study and now produced by Hycrete, Inc. A fourth system, in which the epoxy coating Scotchkote™ 413 was applied to the bars after the application of a primer coating that contains microencapsulated calcium nitrite, was also evaluated. According to 3M™, the system provides protection by releasing calcium nitrite as the epoxy coating is damaged. The systems are identified, respectively, as ECR(DCI), ECR(HY), ECR(RH), and ECR(primer/Ca(NO2)2). The three corrosion inhibitors (DCI® S, Hycrete™, and Rheocrete®) were also studied in conjunction with conventional uncoated reinforcement and are identified as Conv.2(DCI), Conv.2(HY), and Conv.2(RH), respectively.
The three types of ECR with improved adhesion were also evaluated in mortar and concrete containing the corrosion inhibitor calcium nitrite. The systems are identified as ECR(Chromate)-DCI, ECR(DuPont)-DCI, and ECR(Valspar)-DCI.
Western Coating developed a patented process for multiple-coated (MC) bars that involves the application of a layer of 98 percent zinc and 2 percent aluminum to reinforcing steel using a thermal spray coating process prior to the application of the epoxy coating. The zinc layer has a nominal thickness of 50 ![]() m (2 mil). Following application of the zinc, the bars in this study were coated with DuPont™ 8-2739 Flex West Blue, a conventional epoxy.
m (2 mil). Following application of the zinc, the bars in this study were coated with DuPont™ 8-2739 Flex West Blue, a conventional epoxy.
One applicator applied the epoxy to the conventional ECR and ECR(Valspar) bars. A second applicator handled the MC and ECR(DuPont) bars while two other applicators individually handled the ECR(Chromate) and ECR(primer/Ca(NO2)2) bars.
Prior to corrosion testing, the bars used in this study were evaluated for coating thickness and number of holidays. The bars were also evaluated for coating adhesion using the cathodic disbondment test in accordance with ASTM A775-04a and ASTM G8-96 (referred to hereafter as ASTM A775 and ASTM G8, respectively).(13,14)
The six types of ECR bars used in this study were evaluated for coating thickness and number of holidays. The results are summarized in table 3. All bars met the coating thickness requirements of ASTM A775 except the bars with the calcium nitrite primer coating, which tended to have larger percentages of coating measurements below 175 and 125 ![]() m (6.89 and 4.92 mil) than the maximum allowable values of 10 and 5 percent, respectively. [1] (13) Only the bars with the calcium nitrite primer coating exhibited holidays, although the number of holidays was below the maximum allowable of 3 holidays/m (1 holiday/ft) specified in ASTM A775. The other five bar types exhibited no measurable holidays on full-size bars. Additional tests of small bar samples, however, indicated the presence of a small number of holidays on all bar types.
m (6.89 and 4.92 mil) than the maximum allowable values of 10 and 5 percent, respectively. [1] (13) Only the bars with the calcium nitrite primer coating exhibited holidays, although the number of holidays was below the maximum allowable of 3 holidays/m (1 holiday/ft) specified in ASTM A775. The other five bar types exhibited no measurable holidays on full-size bars. Additional tests of small bar samples, however, indicated the presence of a small number of holidays on all bar types.
| Type of Bar | Number of Measurementsa | Maximum ( | Minimum ( | Average ( | Coefficient of Variation |
|---|---|---|---|---|---|
ECR | 4 bars, 144 locations | 307 | 175 | 244 | 0.10 |
ECR(Chromate) | 1 bar, 36 locations | 241 | 175 | 213 | 0.07 |
ECR(DuPont) | 1 bar, 36 locations | 249 | 160 | 213 | 0.08 |
ECR(Valspar) | 1 bar, 36 locations | 262 | 175 | 226 | 0.11 |
ECR(primer/ Ca(NO2)2) | 4 bars, 126 locations | 264 | 102 | 188 | 0.16 |
MC | 1 bar, 36 locations | 251 | 213 | 236 | 0.04 |
1 ![]() m = 0.0394 mil
m = 0.0394 mil
a Bars were 6.1 m (20 ft) long; 15 to 18 measurements were evenly spaced along each side of the test bar.
Cathodic disbondment tests involve the penetration of the epoxy coating on a test specimen using a 3-mm (0.12-inch)-diameter drill bit. The specimen is then immersed for 168 h in an electrolyte (3 percent sodium chloride) solution at 24 ±2 °C (75 ±3.6 °F) and maintained at a -1.5 V potential difference with an anode, measured with respect to a saturated calomel electrode (SCE). The test setup, as described in ASTM A775, is shown in figure 1.(13)
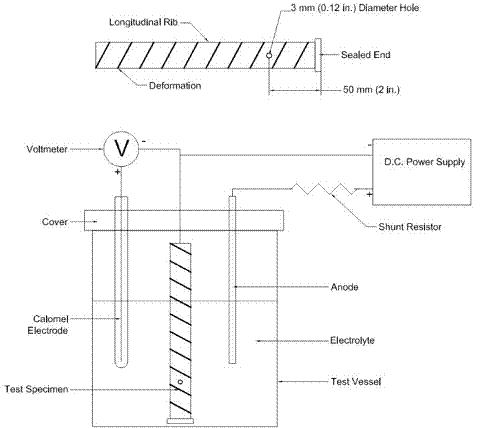
Figure 1. Illustration. Cathodic disbondment test setup (after reference 13).
An examination is performed immediately upon termination of the test. At the end of the test period, the test area is rinsed with warm tap water. The sample is immediately wiped dry, and the entire area of coating is visually examined at the edge of the intentional defect. A new defect, to serve as a reference, is drilled in a portion of the coated area that was not immersed. Two radial cuts at 90 degrees to each other and oriented 45 degrees with respect to the longitudinal axis of the bar are made through the coating, intersecting the center of both intentional defects, using a sharp, thin-bladed knife. An attempt is then made to lift the coating at both the reference defect and the submerged defect with the point of the knife. The bond at the reference defect is then used to judge the quality of the bond at the submerged holiday. Finally, the increase in radial area and total area of the disbonded coating at the submerged defect are measured and recorded.
In this study, three rounds of cathodic disbondment tests were performed, with one specimen per round for each of the six types of ECR. Tests were also performed on conventional epoxy-coated bars that had been used in a previous study. In accordance with ASTM A775, four radial measurements were taken of the disbonded region at 0, 90, 180, and 270 degrees with respect to the longitudinal axis of the bar, and the values were averaged.(13) The cathodic disbondment tests were recorded in terms of both the area of the disbonded coating (in accordance with ASTM G8) and the average coating disbondment radius (four measurements).(14) The results are summarized in table 4. The area of the disbonded coating and the radius do not include the original penetration through the coating. As shown in table 4, the average coating disbondment radius was above 4 mm (0.16 inches), the maximum allowed in annex A1 of ASTM A775, for the conventional ECR and ECR(Valspar) bars, indicating that these bars failed the coating disbondment requirements. The MC reinforcement, ECR(DuPont), ECR(Chromate), and ECR(primer/Na(NO2)2) bars met the coating disbondment requirements. Table 4 also shows that the conventional ECR exhibited the highest area of disbonded coating, with an average value of 178 mm2 (0.271 in2). The high-adhesion Valspar® bars had an area of disbonded coating of 151 mm2 (0.230 in2), followed by ECR with calcium nitrite primer at 67 mm2 (0.102 in2) and the high-adhesion DuPont™ bars at 65 mm2 (0.099 in2). The MC and ECR(Chromate) bars had the lowest areas of disbonded coating, with average values of 27 and 20 mm2 (0.041 and 0.030 in2), respectively. Like the conventional ECR used in this study, the conventional ECR from the previous study also exhibited an average disbondment radius, 5.5 mm (0.21 inches), that exceeded the 4 mm (0.16 inches) allowed by ASTM A775; the disbonded area equaled 168 mm2 (0.256 in2).
Table 4. Cathodic disbondment results
| Type of Bar | Test No. | Thickness ( | Coating Disbondment Radiusa (mm) | Area of Disbonded Coatingb (mm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 0o | 90o | 180o | 270o | Average | ||||
ECR | 1 | NM | 6.5 | 6.5 | 6 | 5.5 | 6.1 | 183 |
2 | NM | 6.5 | 5 | 3.5 | 4 | 4.8 | 133 | |
3 | 249 | 6.5 | 6.5 | 7.5 | 6.5 | 6.8 | 219 | |
Average | 5.9 | 178 | ||||||
ECRc | 1 | 300 | 5.5 | 6.5 | 5.5 | 5 | 5.6 | 170 |
2 | 274 | 5.5 | 4.5 | 4.5 | 5.5 | 5.0 | 161 | |
3 | 241 | 6.5 | 5.5 | 5.5 | 5.5 | 5.8 | 174 | |
Average | 5.5 | 168 | ||||||
ECR(Chromate) | 1 | NM | 0.5 | 1 | 0 | 0 | 0.4 | 6 |
2 | NM | 1 | 0.5 | 2 | 2.5 | 1.5 | 35 | |
3 | 279 | 1.5 | 0.5 | 0.5 | 1.5 | 1.0 | 19 | |
Average | 1.0 | 20 | ||||||
ECR(DuPont) | 1 | NM | 4 | 3 | 3.5 | 3.5 | 3.5 | 93 |
2 | NM | 1.5 | 1 | 1.5 | 1 | 1.3 | 19 | |
3 | 224 | 3.5 | 4 | 3.5 | 4 | 3.8 | 83 | |
Average | 2.8 | 65 | ||||||
ECR(Valspar) | 1 | NM | 4.5 | 4 | 4.5 | 4 | 4.3 | 133 |
2 | NM | 6 | 4.5 | 5.5 | 4.5 | 5.1 | 167 | |
3 | 269 | 6.5 | 4.5 | 5.5 | 4.5 | 5.3 | 154 | |
Average | 4.9 | 151 | ||||||
ECR | 1 | NM | 1.5 | 2 | 2 | 2 | 1.9 | 58 |
2 | NM | 3.5 | 2.5 | 4.5 | 2.5 | 3.3 | 77 | |
3 | 203 | 3 | 2.5 | 2.5 | 2.5 | 2.6 | 67 | |
Average | 2.6 | 67 | ||||||
MC | 1 | NM | 2.5 | 1.5 | 1 | 1 | 1.5 | 22 |
2 | NM | 2 | 1.5 | 1.5 | 3 | 2.0 | 35 | |
3 | 284 | 0.5 | 2.5 | 1.5 | 1.5 | 1.5 | 25 | |
Average | 1.7 | 27 | ||||||
1 ![]() m = 0.0394 mil
m = 0.0394 mil
1 mm = 0.039 inches
1 mm2 = 0.00155 in2
NM = Not measured
a Coating disbondment radius measured from edge of 3-mm (0.12-inch)-diameter hole.
b Area of disbonded coating is the total area after disbondment minus the original area of a 3-mm (0.12-inch)-diameter hole.
c Conventional ECR from previous study.
The criteria in annex A1 of ASTM A775 are qualification requirements for the epoxy coating itself and are not meant to be applied to production bars such as those used in this study. Thus, although all bars did not all meet the qualification criteria, they are considered to be representative of bars used in practice. Further, as observed in earlier tests by McDonald et al., the performance of the bars in the cathodic disbondment tests did not prove to be a predictor of their performance in the corrosion tests in this study.(16)
The corrosion-protection systems in this study were evaluated using a combination of laboratory and field tests. The performance of each system was compared to that of conventional ECR and uncoated mild steel reinforcement. The tests included rapid macrocell tests, bench-scale tests, linear polarization resistance, and field tests.
Summary of Method
The response of the multiple corrosion-protection systems was first evaluated using the rapid macrocell test, originally developed at the University of Kansas under the Strategic Highway Research Program and since updated. (See references 17 through 26). The goal of the test is to obtain a realistic measure of the performance of corrosion-protection systems in a short time period. The basic test specimen consists of either a bare reinforcing bar or a bar clad in mortar (mortar-wrapped), as illustrated in figure 2 through figure 4. The procedures used for bare bars are incorporated in annex A2 of ASTM A955.(27) The contact surface between the mortar and the bar simulates the contact obtained between concrete and reinforcing bars in structures through the use of realistic water-cement and sand-cement ratios.
Figure 2. Illustration. Macrocell test with bare bar specimens.
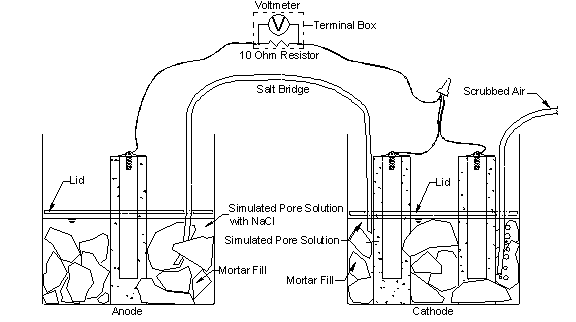
Figure 3. Illustration. Macrocell test with mortar-clad specimens.
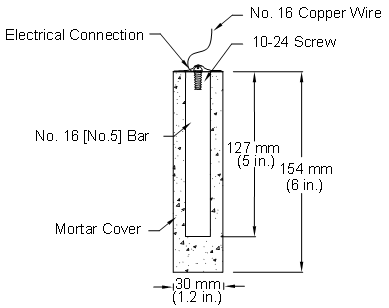
Figure 4. Illustration. Mortar-wrapped specimen containing conventional reinforcing bar.
The macrocell tests shown in figure 2 and figure 3 require two containers. The test specimen, either a bare or mortar-wrapped No. 16 (No. 5) bar, is placed in a 1.5-L (1.6-qt) container along with simulated pore solution containing a preselected concentration of sodium chloride (1.6 or 6.04 moles/kg (0.73 or 2.74 moles/lb) of solvent-ion (4.68 or 15 percent) concentration). Two specimens are placed in a second container and immersed in simulated pore solution with no chlorides added. For mortar-wrapped specimens, crushed mortar fill is added to the containers to more closely simulate the concrete environment. The solution depth exposes 76 mm (3.0 inches) of the bar (including the 13-mm (0.5-inch) plastic cap used to protect the end of epoxy-coated bars) below the level of the solution. The two containers are connected by a salt bridge, and the test specimen in the pore solution containing sodium chloride (anode) is electrically connected across a single 10-ohm resistor to the two specimens in the simulated pore solution (cathode). The resistors are mounted between binding posts in a terminal box to consolidate the specimen wires. Air (scrubbed to remove carbon dioxide) is bubbled into the liquid surrounding the cathode to ensure an adequate supply of oxygen. Plastic lids are placed just above the surface of the solution to hold the specimens in place and reduce evaporation of the solution. Holes are cut in the lids to introduce the specimens, a salt bridge, and the air supply. The air causes some evaporation, which is countered by adding deionized water to the container to maintain a constant volume of solution. The solutions in both containers are changed once every 5 weeks to further protect against carbonation.[2] The corrosion current and the rate of corrosion are determined by measuring the voltage drop across the resistor. The open circuit corrosion potentials of the cathode and anode are also measured with respect to an SCE after the circuit has been open for 2 h to allow the potentials to stabilize. The simulated pore solution, consisting of sodium hydroxide and potassium hydroxide, matches that obtained in a pore solution analysis.(28,29) Epoxy-coated steel is evaluated using specimens in which the coating is breached by four 3.2-mm (0.13-inch)-diameter holes to simulate defects in the epoxy coating (damaged area equals 1.0 percent of total exposed area in the solution). In the rapid macrocell test, bare conventional bars exhibit corrosion initiation within the first 24 h, and conventional bars cast in mortar exhibit corrosion within the first week. The tests last 15 weeks.
Corrosion Rate and Corrosion Loss
The corrosion rate of reinforcing steel (measured in the bench-scale and field tests as well as in the rapid microcell tests) indicates how fast reinforcing steel is oxidized. It may be expressed as a current density in microamps per square centimeter (![]() A/cm2), which is obtained by measuring the rate of electron flow from anodes to cathodes. Based on Faraday's law, current density can be converted to another expression for corrosion rate, a rate of loss of metal from the surface of the steel in
A/cm2), which is obtained by measuring the rate of electron flow from anodes to cathodes. Based on Faraday's law, current density can be converted to another expression for corrosion rate, a rate of loss of metal from the surface of the steel in ![]() m/year.(30) The equation in figure 5 shows this conversion for iron.
m/year.(30) The equation in figure 5 shows this conversion for iron.
![]()
Figure 5. Equation. Corrosion rate for iron.
Where:
R = Corrosion rate, given as rate of metal loss, ![]() m/year.
m/year.
i = Corrosion rate, given as current density, ![]() A /cm2.
A /cm2.
k = Conversion factor = 31.5 '104 amp∙![]() m s/
m s/![]() A cm∙year.(30)
A cm∙year.(30)
a = Atomic weight of the metal = 55.8 g/mole for iron.
n = Number of electrons transferred = 2 for iron.
F = Faraday's constant = 96,500 Coulombs/equivalent.
![]() = Density of the metal, g/cm3 = 7.87 g/cm3 for iron.
= Density of the metal, g/cm3 = 7.87 g/cm3 for iron.
For example, calculating corrosion loss for a bare conventional bar, a voltage drop of 0.70 mV across the 10‑ohm resistor represents a total current of 70.0 ![]() A. For a No. 16 (No. 5) bar and a solution depth of 76 mm (3.0 inches) (the values used in the test), the total surface area in contact with the solution (based on the nominal diameter of the bar and including the end of the bar) is 4,021 mm2 (6.233 in2), giving a current density i of 1.74
A. For a No. 16 (No. 5) bar and a solution depth of 76 mm (3.0 inches) (the values used in the test), the total surface area in contact with the solution (based on the nominal diameter of the bar and including the end of the bar) is 4,021 mm2 (6.233 in2), giving a current density i of 1.74 ![]() A/cm2 (11.2
A/cm2 (11.2 ![]() A/in2). Applying the equation in figure 5 results in the equation in figure 6.
A/in2). Applying the equation in figure 5 results in the equation in figure 6.
![]()
Figure 6. Equation. Example of corrosion rate for iron.
For zinc, the first coating layer on the MC bars, the equation in figure 5 becomes the equation in figure 7.
![]()
Figure 7. Equation. Corrosion rate for zinc.
Where:
a = Atomic weight of the metal = 65.38 g/mole for zinc.
n = Number of electrons transferred = 2 for zinc.
![]() = Density of the metal, g/cm3 = 7.14 g/cm3 for zinc.
= Density of the metal, g/cm3 = 7.14 g/cm3 for zinc.
For reinforced concrete bridge decks, the measurement of the macrocell current is generally not possible because the top and bottom mats of reinforcing steel are usually connected by steel wire ties and bar supports in the concrete slab. In laboratory tests that simulate the corrosion of steel in bridge decks, however, ties and bar supports are not used, and the macrocell current can be determined by measuring the voltage drop across a resistor that electrically connects the anode and the cathode through an external circuit, as shown in the equation in figure 8.
![]()
Figure 8. Equation. Corrosion current density.
Where:
i = Corrosion current density, mA/cm2.
V = Voltage drop across the resistor, mV.
R = Resistance of the resistor, kilohms.
A = Area of exposed metal on the anode bar, cm2.
The measured macrocell current density and the calculated corrosion rate can be affected significantly by the test methods and the details of the test configuration such as the anode to cathode area ratio and the size of the resistor connecting the anode and the cathode.(31,16) Thus, the corrosion rate calculated from the measured macrocell current should be used only to compare the relative performance of corrosion-protection systems under same test conditions.
Corrosion loss represents cumulative metal loss expressed in micrometers and is calculated by numerically integrating the corrosion rate.
Test Specimens
The specimens in the rapid macrocell test consist of 127-mm (5-inch)-long No. 16 (No. 5) reinforcing bars, either bare or embedded in mortar, as illustrated in figure 9 for epoxy-coated bars.
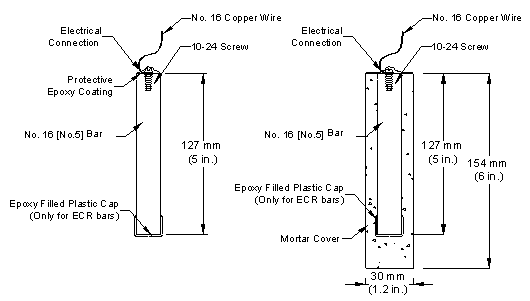
Figure 9. Illustration. Bare bar and mortar-wrapped rapid macrocell specimens with cap to protect the exposed end of epoxy-coated bars.
The following procedure is used to fabricate the specimens:
Test Materials
The following materials are used in rapid macrocell tests:
Data Acquisition
A voltmeter with a 0.001 mV resolution is used to measure corrosion potential of the anode and cathode and the voltage drop across the 10-ohm resistor. In a typical test, the voltage drop tends to fluctuate between -0.003 and 0.003 mV when the corrosion current is close to zero. Only voltage drop readings outside of this region are used to evaluate the performance of the corrosion-protection systems. Values between -0.003 and 0.003 mV are treated as zero.
During the past two decades, bench-scale tests such as the southern exposure, cracked beam, and ASTM G109 tests have frequently been used to evaluate the corrosion performance of reinforcing steel.(31,35,36) Of these tests, the southern exposure and cracked beam tests have proven to give useful data in a relatively short period. All three test methods were used in this study, but only the results for the first two are reported because they yielded useful data. The ASTM G109 test uses only a 3 percent sodium chloride solution and provides a much milder degree of exposure to the specimens. It yielded no useful information over a 4-year period, twice as long as that used for the southern exposure and cracked beam tests.
Southern Exposure Test
The specimen used in the southern exposure test consists of a small slab containing two mats of reinforcing steel (see figure 10).(37)
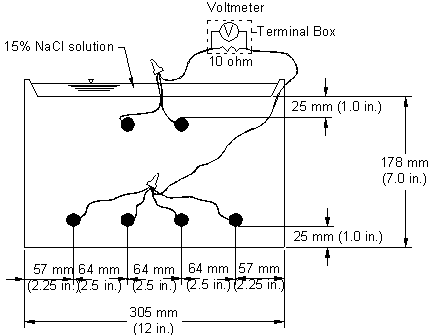
Figure 10. Illustration. Southern exposure test specimen.
The concrete is wet cured for 3 days and then air cured until the test begins at 28 days. The top mat consists of two No. 16 (No. 5) bars, and the bottom mat consists of four No. 16 (No. 5) bars. The mats are connected electrically across a 10-ohm resistor, a dam is placed around the edge of the top surface (cast integrally with the specimen), and the sides of the concrete are sealed with epoxy. A 15 percent (6.04 molal ion) sodium chloride solution is placed inside the dam, allowing the chlorides to penetrate the concrete. The slabs are subjected to a 7-day alternate ponding and drying regime, with ponding at 23 ±2 °C (73 ±3 °F) for 4 days and drying at 38 °C (100 °F) (thus the name southern exposure) for 3 days. Prior to drying, the solution is removed from the upper surface using an industrial vacuum cleaner. The ponding and drying regime continued for 12 weeks. The specimens were then subjected to continuous ponding for 12 weeks at 23 ±2 °C (73 ±3 °F) after which the alternate ponding and drying regime begins again. The two regimes continued for 96 weeks. Corrosion current and the corresponding corrosion rate are determined by measuring the voltage drop across the resistor. The corrosion potentials of the top and bottom bars are measured.[3] Corrosion performance is also evaluated using monthly linear polarization resistance readings on selected specimens. The test provides a severe corrosion environment that is generally believed to simulate 15-20 years of exposure for marine structures under tropical conditions and 30-40 years of exposure for bridges within a 48-week period.(38)
Upon corrosion initiation and at the conclusion of the tests, southern exposure specimens are sampled for chloride content using procedures described in the section on chloride analysis later in this chapter.
Cracked Beam Test
The cracked beam specimen is used to model the corrosion of reinforcing steel in which cracks directly expose the steel to deicing chemicals (see figure 11). The specimen is half the width of the southern exposure specimen, with one bar on top and two bars on the bottom.
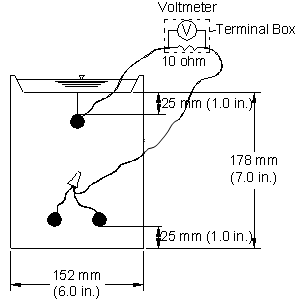
Figure 11. Illustration. Cracked beam test specimen.
A crack is simulated parallel to and above the top reinforcing bar through the insertion of a 0.3‑mm (12‑mil) stainless steel shim when the specimen is fabricated. The shim is removed within 12 h of casting, leaving a direct path for chlorides to the reinforcing steel and simulating the effects of a settlement crack over the bar. An integral dam is used in manner similar to that used for the southern exposure specimen around the upper surface of the specimen. Like the southern exposure specimen, the cracked beam specimen is subjected to cycles of wetting and drying with a 15 percent sodium chloride solution, continuing up to 96 weeks.
Specimen Fabrication
The following process is used to fabricate southern exposure and cracked beam specimens:
| w/c | Cement, kg/m3 | Water, kg/m3 | Fine Aggregate, kg/m3 | Coarse Aggregate, kg/m3 | Air-Entraining Agent, mL/m3 | DCI, L/m3 | Hycrete™, kg/m3 | Rheocrete®, L/m3 | SP, L/m3 |
|---|---|---|---|---|---|---|---|---|---|
0.45 |
355 |
160 |
851 |
880 |
90 |
- |
- |
- |
- |
355 |
147.4 |
851 |
880 |
140 |
15 |
- |
- |
- |
|
355 |
154.0 |
851 |
880 |
35 |
- |
8.0 |
- |
- |
|
355 |
155.7 |
851 |
880 |
300 |
- |
- |
5 |
- |
|
0.35 |
438 |
153 |
764 |
861 |
355 |
- |
- |
- |
2.12 |
438 |
140.4 |
764 |
861 |
740 |
15 |
- |
- |
2.12 |
|
438 |
145.6 |
764 |
861 |
330 |
- |
9.9 |
- |
2.25 |
|
438 |
148.7 |
764 |
861 |
1,480 |
- |
- |
5 |
2.25 |
1 kg/m3 = 1.69 lb/yd3
1 mL/m3 = 0.026 fl oz/yd3
1 L/m3 = 0.202 gal/yd3
SP = Superplasticizer (Rheobuild® 1000 by BASF Admixtures, Inc.)
- Not used.
Test Materials
The properties of the materials were as follows:
A 15 percent sodium chloride solution (6.04 molal ion concentration) was used to pond the test specimens: 600 mL (20.3 fl oz) to pond one southern exposure specimen and 300 mL (10.1 fl oz) to pond one cracked beam specimen.
Data Acquisition
The same voltmeter and rules for data conversion described for the rapid macrocell tests were used for the bench-scale tests.
The corrosion initiation beam test is used to determine the critical chloride corrosion threshold of a corrosion-protection system. While these data are also obtained from southern exposure specimens, corrosion initiation tests are terminated at the onset of corrosion, thus allowing a greater number of samples to be collected. The test was used in this study to evaluate conventional reinforcing steel cast in concrete containing corrosion inhibitors. The specimen is identical to the cracked beam specimen except that no intentional crack is placed above the reinforcement. The corrosion initiation beam specimen is shown in figure 12.
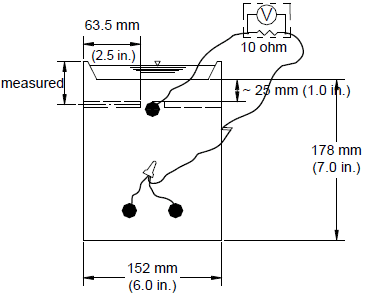
Figure 12. Illustration. Corrosion initiation beam test specimen.
The materials, fabrication, and testing procedures used in the corrosion initiation tests are identical to those used for the southern exposure and cracked beam tests with the exception of test duration. The w/c ratio is 0.45.
For conventional reinforcing steel, corrosion initiation is considered
to occur when either the macrocell corrosion
rate first reaches a value greater than or equal to 0.3 ![]() m/year (0.01 mil/year) or when the corrosion
potential of the top mat of steel first shifts to a value more negative than ‑0.350
V with respect to a CSE. For zinc-coated steel, corrosion initiation is based
on a corrosion rate of 0.3
m/year (0.01 mil/year) or when the corrosion
potential of the top mat of steel first shifts to a value more negative than ‑0.350
V with respect to a CSE. For zinc-coated steel, corrosion initiation is based
on a corrosion rate of 0.3 ![]() m/year (0.01 mil/year) or when a sharp
change in corrosion potential is observed, with the former serving as the
primary guide.
m/year (0.01 mil/year) or when a sharp
change in corrosion potential is observed, with the former serving as the
primary guide.
Once corrosion initiation occurs, testing is halted and concrete samples are taken for chloride analysis. A total of 20 samples are taken from each initiation beam, 10 from each side of the beam starting 40 mm (1.5 inches) from the edge and spaced at 25-mm (1-inch) intervals, as shown in the side view of the specimen in figure 13. For each sample, drilled holes are positioned so that the top of the holes and the top surface of the reinforcing bar are at the same level. To do this, the actual level of the top reinforcing bar in each specimen is measured. Concrete sampling and chloride analysis are preformed as described later in this chapter.
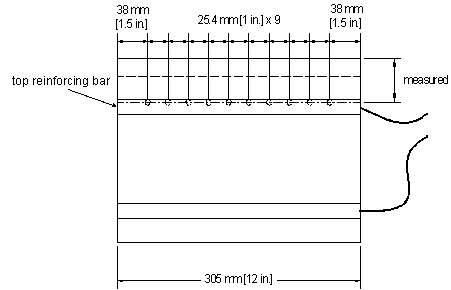
Figure 13. Illustration. Sampling locations for initiation beam tests.
A measure of both microcell and macrocell corrosion can be obtained with the polarization resistance test, which uses a noncorroding counter electrode and a reference electrode to establish a polarization curve by imposing a range of potentials on the metal and measuring the corresponding corrosion currents using a potentiostat. Polarization resistance measurements were obtained from selected bench-scale specimens throughout the test period.
Polarization resistance tests were used in this study to obtain the total corrosion rates for bench-scale specimens. In the tests, current readings are taken during a short, slow sweep of bar potential. The sweep typically ranges from -20 to +20 mV relative to the open circuit potential Eoc. In this range, the current-versus-voltage curve is roughly linear. The slope of the linear region is proportional to the resistance of the metal. The total corrosion current density is obtained using the relationship shown in figure 14.
![]()
Figure 14. Equation. Corrosion current density.
Where:
i = Corrosion current density (A/cm2).
B = Stern-Geary constant (typically taken as 26 mV for both reinforcing steel and zinc in concrete).
Rp = Slope determined from the polarization curve (kilohms'cm2).
The total corrosion rate in ![]() m/year is calculated using the
equations in figure 5 and figure 7 for iron and zinc, respectively. In this
study, the tests are performed using a PC4/750 Potentiostat and DC105 corrosion
measurement system from Gamry Instruments.
m/year is calculated using the
equations in figure 5 and figure 7 for iron and zinc, respectively. In this
study, the tests are performed using a PC4/750 Potentiostat and DC105 corrosion
measurement system from Gamry Instruments.
The tests for bench-scale specimens were performed every 4 weeks to obtain the total corrosion rates for the top mats with the bottom mats disconnected. In the tests, the top mat of reinforcing steel is used as the working electrode, an SCE immersed in salt solution on top of the specimen is used as the reference electrode, and a platinum strip immersed in the salt solution is used as the counter electrode.
The data file from a polarization resistance test is analyzed using the data analysis package provided with the DC105. This analysis software can read the data file and plot a graph based on the data in the file. When a new graph is created in this package, the user picks a range of voltage in the graph and the software automatically uses a linear fit of the data in the selected range to calculate the polarization resistance. The corrosion current density and corrosion rate can then be determined using the polarization resistance.
Using concrete slab test specimens stored outdoors, the field test is designed to obtain a measure of the performance of corrosion-protection systems under realistic exposure conditions. Like the bench-scale specimens, some field test specimens are uncracked and some have simulated cracks directly above and parallel to selected reinforcing bars. A dam made of weatherstripping is attached to the upper concrete surface to hold a salt solution that is ponded on the specimens every 4 weeks. Corrosion rate measurements are obtained for a minimum of 250 weeks.
Field test specimens consisted of a 1,219 x 1,219 x 165-mm (48 x 48 x 6.5-inch) concrete slabs with two mats of No. 16 (No. 5) reinforcing bars (see figure 15 through figure 18). Each mat contains seven bars in both the longitudinal and transverse directions with clear concrete covers 25 mm (1 inch) from the top and bottom and 76 mm (3 inches) from the ends. Selected top and corresponding bottom bars are electrically connected across a 10-ohm resistor to form one test point.
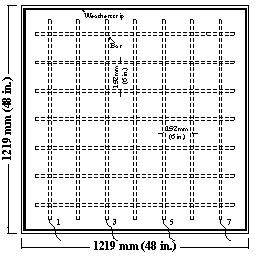
Figure 15. Illustration. Field test specimens, top slab without cracks.
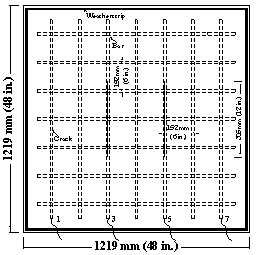
Figure 16. Illustration. Field test specimens, top slab with cracks.
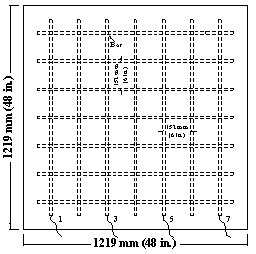
Figure 17. Illustration. Field test specimens, bottom slab.
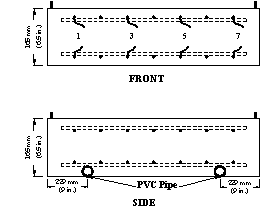
Figure 18. Illustration. Field test specimens, front and side views.
Test specimens are moved to the Adams Campus of the University of Kansas 7 days before testing. The specimens are spaced 0.914 m (3 ft) apart and placed 203 mm (8 inches) above the ground using 203 x 203 x 406-mm (8 x 8 x 16-inch) concrete blocks.
A 9.5-mm (0.375-inch)-thick dam made of weatherstrip tape is attached to the top concrete surface around the edges and sealed with silicone caulk to prevent leakage. The specimens are ponded with 3.3 L (0.87 gal) of 10 percent rock salt solution, which contains 0.30 kg (0.66 lb) of rock salt, every 4 weeks. Occasionally in winter, rock salt alone is used.
The salt exposure program was based on Kansas salt usage history from 1998 to 2002, as shown in table 6.(40) The average application rate was based on a total length of all driving lanes of 33,742 km (20,967 mi) with an average lane width of 3.7 m (12 ft). The yearly average salt application was 0.66 kg/m2 (0.13 lb/ft2).
Table 6. Kansas Department of Transportation (KDOT) salt usage history.(40)
| Fiscal Year | Rock Salt Total | Average Application Rate |
||
|---|---|---|---|---|
| Metric Ton | Ton | kg/m2 | lb/ft2 | |
1998 |
86,507 |
95,374 |
0.71 |
0.14 |
1999 |
64,254 |
70,840 |
0.52 |
0.11 |
2000 |
58,583 |
64,588 |
0.48 |
0.1 |
2001 |
124,619 |
137,392 |
1.02 |
0.21 |
2002 |
67,673 |
74,609 |
0.55 |
0.11 |
Average |
80,327 |
88,561 |
0.66 |
0.13 |
The KDOT Maintenance Manual provides general guidelines for salt applications in snow season.(41) According to KDOT personnel, bridge decks receive four to five times the amount of salt applied to the adjacent pavement to account for lower temperatures on bridge decks. To match the approximate quantity of salt applied to bridge decks, four times the application rate of salt on pavements in Kansas, which is 2.64 kg/m2 (0.52 lb/ft2), is applied to field test specimens. This translates to 3.92 kg (8.32 lb) per specimen per year or 0.30 kg (0.66 lb) of rock salt every 4 weeks, the value used in the tests, as described above.
The 16-gauge copper wires from the top mat bars are connected to red binding posts, and the wires from the bottom mat bars are connected to paired black binding posts. A switch was connected to the red binding post through a 10-ohm resistor. The switches are turned on and off to control the electrical circuits. Binding posts of several specimens are centered together in a terminal box for testing convenience.
The test specimens are ponded with 3.3 L (0.87 gal) of 10 percent rock salt solution on the first day. Two weeks later, the voltage drops across the 10-ohm resistors are measured using a voltmeter. The circuits are then opened and the mat-to-mat resistances are recorded using an ohmmeter. The corrosion potentials are measured about 2 h after opening the circuits. Both anode and cathode corrosion potentials are measured with respect to a CSE. The circuits are closed after all the readings are taken. To achieve consistent measurements, specimens are watered before taking readings, usually about an hour before voltage drops.
Corrosion potential varies with temperature, and temperatures fluctuate in the field.(42) Therefore, a correction factor must be applied to convert potential measurements taken in the field to a value corresponding to 22° C (72° F). For a CSE, the correction is shown in figure 19 and figure 20.
![]()
Figure 19. Equation. Temperature correction in Celsius.
![]()
Figure 20. Equation. Temperature correction in Fahrenheit.
Where:
T = Temperature, °C or °F.
Eo = Uncorrected corrosion potential reading, mV.
E = Temperature corrected corrosion potential reading, mV.
The test cycle is repeated every 4 weeks. The specimens are ponded at the same time readings are taken. After the specimens reach about 96 weeks, readings are taken every 8 weeks, but the ponding cycle is maintained at 4 weeks.
Corrosion potentials are measured at fixed grid points on the top specimen surface, as shown in 21 through figure 23.
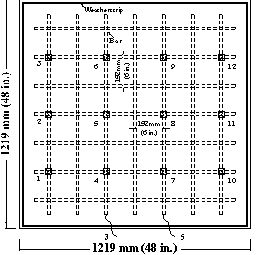
Figure 21. Illustration. Potential test points for conventional steel specimens.
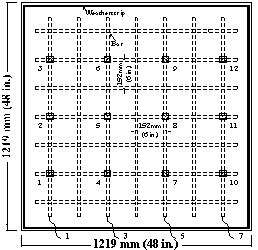
Figure 22. Illustration. Potential test points for epoxy-coated bar specimens with four test bars.
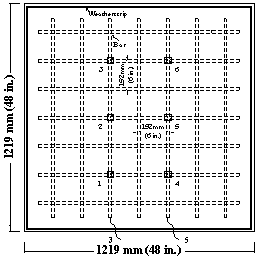
Figure 23. Illustration. Potential test points for epoxy-coated bar specimens with two test bars.
Specimen Fabrication
The following process is used to fabricate field test specimens:
A 914-mm (36-inch)-long 14-gauge electrical copper wire is connected to the tapped end of the test bars with a 10-mm (0.375-inch) 10-24 stainless steel threaded bolt. The connection and all other exposed ends of epoxy-coated and MC bars are coated with 3M™ Rebar Patch epoxy. After the epoxy dried, a 76-mm (3-inch)-long heat-shrinkable tube is used to protect and direct the copper wire out of the specimen. Because 76 mm (3 inches) of the bar is covered by the tube, the holes in the epoxy represent 0.26 percent of the exposed bar surface. The interface between the shrinkable tube and the tapped end is patched with epoxy. As shown in figure 15 through figure 18, bars numbered 1, 3, 5, and 7 are connected across 10-ohm resistors. In some early test specimens, only bars 3 and 5 were connected.
Plastic chairs 25-mm (1-inch)-high are used to support bottom mat bars, and 108-mm (4.25-inch)-high plastic chairs are used to support top mat bars to provide 25-mm (1‑inch) clear concrete covers. Plastic rather than metal chairs are used to avoid unplanned electrical connections between the top and bottom bars. The steel within each mat is connected using conventional tie wire for conventional steels and plastic-coated tie wire for epoxy-coated steels.
For specimens with simulated cracks from the top surface, 0.3-mm (12-mil)-thick, 305‑mm (12-inch)-long stainless steel shims are placed directly above and parallel to the test bars. The crack thickness is designed based on bridge deck crack surveys.(43) A shim holder is used to position stainless steel shims in place. It is made of plywood and structured as shown in figure 24 through figure 26. The stainless steel shims are attached to the top of the shim holder and the holder is attached into the form using wood screws.
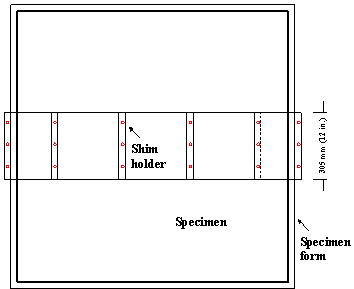
Figure 24. Illustration. Shim holder for field specimens, top view.
Figure 25. Illustration. Shim holder for field specimens, front view.
Figure 26. Illustration. Shim holder for field specimens, side view.
Concrete is consolidated during casting using an electric internal vibrator with a 33-mm (1.375-inch)-diameter head. The upper surface is finished with a screed, followed by a bullfloat. For cracked specimens, a wooden float is used instead of a bullfloat. The stainless steel shims are removed from the concrete about 12 h after casting.
During casting, concrete slump, temperature, unit weight, and air content are measured in accordance with ASTM standards. Test cylinders are made for each batch and stored with the specimens in a curing room and in a curing tank containing lime-saturated water. The cylinders are tested at 28 days to determine compressive strength. Concrete batch information and the properties of the plastic and hardened concrete are summarized in table 7. As shown in the table, the concrete in the specimens containing Rheocrete® and Hycrete™ exhibited reduced compressive strength compared to other specimens independent of w/c ratio. The reduced strengths may be explained in part by analyses of pore solutions in cement pastes by O'Reilly et al., who observed a marked increase in sulfate content at 7 days for pastes containing Rheocrete® and at both 1 and 7 days for cement pastes containing Hycrete™.(44) These increases in sulfate content may also explain differences in the critical chloride corrosion threshold for concretes containing corrosion inhibitors, as discussed in chapter 3.
Table 7. Properties of concrete batches for field tests.
| Batch | Steel Designationa (No. of Specimens) | w/c | Slump, mm | Concrete Temp., °C | Unit Weight, kg/m3 | Air Content, Percent | Average Compressive Strengthb, MPa | ||
|---|---|---|---|---|---|---|---|---|---|
| Curing Tank | Curing Room | With Specimens | |||||||
1 |
Conv. (2), ECR (2), ECR(Valspar) (2) |
0.39 |
100 |
19 |
2,219 |
6.25 |
- |
28.4 |
30.6 |
2 |
ECR(DuPont) (2), ECR(Chromate) (2), MC (2) |
0.43 |
100 |
19 |
2,319 |
5.00 |
- |
35.7 |
37.4 |
3 |
ECR(primer/Ca(NO2)2) (2), Conv. (2), ECR (2) |
0.41 |
50 |
28 |
2,307 |
4.00 |
- |
34.4 |
36.9 |
4 |
ECR(Valspar) (2), ECR(DuPont) (2), ECR(Chromate) (2) |
0.42 |
125 |
25 |
2,296 |
5.75 |
- |
32.5 |
32.9 |
5 |
MC (2), ECR(primer/Ca(NO2)2) (2) |
0.44 |
110 |
23 |
2,291 |
5.25 |
32.8 |
32.6 |
33.2 |
6 |
ECR(DCI) (4) |
0.48 |
210 |
22 |
2,255 |
7.25 |
35.3 |
30.9 |
29.6 |
7 |
ECR(DCI) (2) |
0.40 |
25c |
21 |
- |
5.50 |
36.8 |
35.9 |
- |
8 |
ECR(RH) (4) |
0.44 |
165 |
23 |
2,295 |
5.50 |
29.1 |
28.5 |
28.1 |
9 |
ECR(HY) (4) |
0.41 |
185 |
16 |
2,216 |
5.65 |
15.0 |
13.5 |
13.1 |
1 mm = 0.039 inches
°F = 1.8 °C + 32
1 kg/m3 = 1.69 lb/yd3
1 MPa = 145 psi
- Not used.
a See table 1 for abbreviation definitions; MC bars have both zinc
and epoxy layers penetrated; all epoxy-coated bars are penetrated with 16
surface holes.
b Average of three cylinders.
c A slump of 150 mm (6 inches) was measured at the ready-mix plant
before transporting to the concrete lab.
The sampling and testing procedures to determine chloride ion concentration in bench-scale and corrosion initiation specimens were those adopted by Ji et al.(26) Pulverized concrete samples are obtained by drilling 6.4-mm (0.25-inch)-diameter holes in the side of the specimen using a rotary impact-type drill. Prior to sampling, the drilled concrete surface is cleaned three times, first using soap and water, then using tap water, and finally using deionized water. The surface is then dried using paper towels. Drilling positions are measured and marked. A 152-mm (6-inch)-long, 6.4‑mm (0.25-inch)-diameter drill bit is mounted to a heavy-duty drill. The specimen is drilled perpendicular to the reinforcing steel, parallel to the top surface of the specimen. The sample obtained from the first 13-mm (0.5-inch) depth contains epoxy coating from the exterior of the specimen and is discarded. The drilling continues to a depth of 63.5 mm (2.5 inches) to obtain approximately 4 g (0.15 oz) of powder. The pulverized concrete sample from each hole is collected using two pieces of copy paper and transferred to labeled plastic bags. The drill bit is cleaned before and between each sample to avoid contamination. A shop vacuum reserved for drilling is used during the procedure.
Four cores are taken from most field test specimens at end of life using an 89-mm (3.5-inch)-diameter core drill bit and core drill to determine the chloride ion concentration. The cores are taken at the corners of the specimen, 230 mm (9 inches) from the edges. Cores that include reinforcement are not analyzed to avoid measuring the effect of chloride buildup over the bars. After coring, cores are stored at -18○ C (0○ F) to minimize chloride ion migration prior to sampling. Concrete powder for sampling is obtained from the cores using a milling machine and a 32-mm (1.25-inch)-diamond grit hole saw. The cores are mounted on a rotary table and the hole saw is positioned off center from the core bit so that when the rotary table is rotated the hole saw cuts a 44-mm (2.5-inch)-diameter circle in the core. This path avoids sampling from the edge of the core, where water from the core drill may affect the chloride content. Samples are obtained from different depths so that a depth profile of chloride content can be determined. The powder obtained from the top 4 mm (0.15 inches) of the core is discarded because of possible contamination from the core drill water. Samples are collected from 4-8 mm (0.15-0.30 inches), 8-13 mm (0.3-0.5 inches), 13-19 mm (0.5-0.75 inches), 19-25 mm (0.75-1.0 inches), 25-29 mm (1-1.125 inches), 29-32 mm (1.125-1.25 inches), and 38-41 mm (1.5-1.625 inches) with the aid of a vacuum filter collection system.
Concrete samples are analyzed for water-soluble chloride content using procedure A from AASHTO T 260-94.(46) Samples are boiled in deionized water to free any water-soluble chlorides. The solution is filtered, acidified with nitric acid, and titrated with silver nitrate. The potential of a chloride ion-selective electrode is monitored throughout the titration. The change in potential with respect to the volume of silver nitrate is calculated, with the endpoint indicated by the inflection point of the potential-volume curve-the point at which the greatest change in potential for a given incremental addition of silver nitrate was observed. This procedure gives the chloride ion concentration in terms of percent chloride by weight of concrete. In this study, values are presented in kg/m3 (lb/yd3) by multiplying by the unit weight of concrete, taken as 2,246 kg/m3 (3,786 lb/yd3).
Corrosion-protection systems are typically compared based on
average losses of metal from the surface (expressed as ![]() m) over time for a
given test method. These losses are a function of both the time to corrosion
initiation and the corrosion rate following initiation. General comparisons do not necessarily require that the time of
corrosion initiation be precisely indentified. To develop an estimate of the
corrosion performance of a system in a structure such as a bridge deck,
however, requires separate estimates for the time to corrosion
initiation and the subsequent corrosion rate. Because
corrosion rates fluctuate and each specimen is unique, using the combined
average losses versus time underestimates both the average time to
initiation (losses appear to increase at the earliest
initiation time) and the average corrosion rate (losses start slowly because
not all specimens are corroding). To rectify this, bench-scale and field
test specimens in this study are analyzed individually and the results are
combined to determine the average corrosion rate for each system and specimen
type.
m) over time for a
given test method. These losses are a function of both the time to corrosion
initiation and the corrosion rate following initiation. General comparisons do not necessarily require that the time of
corrosion initiation be precisely indentified. To develop an estimate of the
corrosion performance of a system in a structure such as a bridge deck,
however, requires separate estimates for the time to corrosion
initiation and the subsequent corrosion rate. Because
corrosion rates fluctuate and each specimen is unique, using the combined
average losses versus time underestimates both the average time to
initiation (losses appear to increase at the earliest
initiation time) and the average corrosion rate (losses start slowly because
not all specimens are corroding). To rectify this, bench-scale and field
test specimens in this study are analyzed individually and the results are
combined to determine the average corrosion rate for each system and specimen
type.
To illustrate the process, the plots of macrocell corrosion loss versus time for the individual bars in the two field test specimens with conventional steel bars in uncracked concrete containing Rheocrete®, ECR(RH)-U-1 and ECR(RH)-U-2, are shown in figure 27. To determine the average corrosion rate for each bar, the point at which the corrosion loss begins to increase steadily is determined and marked, as shown by red arrows in the figure. The average corrosion rate for the bar is the slope of the line connecting this to the point representing the corrosion loss at the end of the test (see figure 28). The individual averages are combined to obtain the average for the system. Bars that show no increase in corrosion loss, such as ECR(RH)-U-2 (3), are excluded from the average.
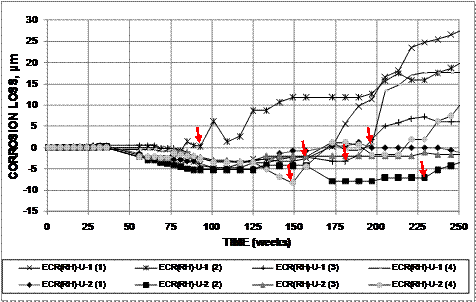
Figure 27. Graph. Individual corrosion losses based on total area of the top bars for field test specimens containing Rheocrete® in uncracked concrete, with corrosion initiation marked.
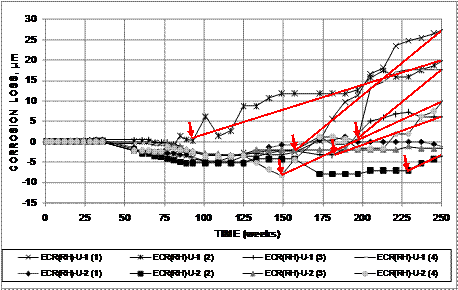
Figure 28. Graph. Individual corrosion losses based on total area of the top bars for field test specimens containing Rheocrete® in uncracked concrete, with lines connecting corrosion loss at initiation to corrosion loss at 250 weeks.
As shown in figure 29 for three southern exposure specimens containing epoxy-coated bars (with four holes through the epoxy) cast in concrete with a w/c ratio of 0.45 containing corrosion inhibitor DCI, some specimens exhibit corrosion loss (the result of short periods of measureable corrosion) and extended periods with no corrosion without having a measureable corrosion rate over time. In figure 29, the corrosion rate after corrosion initiation can be measured for only one of three specimens (ECR-DCI-4h-45-2).
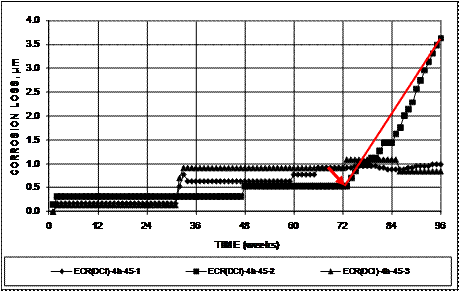
Figure 29. Graph. Individual corrosion loss based on exposed area of the top bars for southern exposure specimens containing epoxy-coated bars cast in concrete containing DCI, with lines connecting corrosion loss at initiation to corrosion loss at 96 weeks.
The test program, summarized in table 8, compared the corrosion performance of the 11 multiple corrosion-protection systems with that of conventional reinforcing steel and conventional ECR and compared the effectiveness of corrosion inhibitors when used with conventional reinforcing steel. As shown in the table, rapid macrocell and bench-scale tests were used for all multiple corrosion-protection systems, but all versions of the tests were not used for every system. In all, the work included 126 macrocell tests, 117 southern exposure tests, 93 cracked beam tests, 30 corrosion initiation beams, 42 field tests, and 32 corrosion-loss-to-crack-concrete tests.
Table 8. Number of test specimens in test program.
| System | Test | ||||
|---|---|---|---|---|---|
| Macrocell | SEb | CBb | FTSc | ||
| Bare | Mortar-wrappeda | ||||
Control |
|||||
Uncoated barsd |
6 |
6 |
15e |
9 |
4f |
ECR |
6 |
6 |
18e,g |
9 |
4f |
Epoxies with increased adhesion |
|||||
Chromate pretreatment |
6 |
6 |
6 |
6 |
4f |
DuPont coating |
6 |
6 |
6 |
6 |
4f |
Valspar coating |
6 |
6 |
6 |
6 |
4f |
Corrosion inhibitors in mortar or concrete |
|||||
Uncoated bars with calcium nitrited |
- |
- |
3 |
3 |
- |
Uncoated bars with Rheocrete® 222+ d |
- |
- |
3 |
3 |
- |
Uncoated bars with Hycrete™ d |
- |
- |
3 |
3 |
- |
ECR with calcium nitrite |
- |
6 |
9g |
9g |
6f |
ECR with Rheocrete® 222+ |
- |
6 |
9g |
9g |
4f |
ECR with Hycrete™ |
- |
6 |
9g |
9g |
4f |
3M™ primer containing calcium nitrite |
- |
6 |
9g |
9g |
4f |
Epoxies with increased adhesion plus calcium nitrite in mortar or concrete |
|||||
Chromate pretreatment |
- |
6 |
3h |
- |
- |
DuPont™ coating |
- |
6 |
3h |
- |
- |
Valspar® coating |
- |
6 |
3h |
- |
- |
Bars with multiple coatings |
|||||
Both layers penetrated |
6 |
6 |
6 |
6 |
4f |
Epoxy only penetrated |
6 |
6 |
6 |
6 |
- |
SE = southern exposure; CB = cracked
beam; FTS = field test specimen
- No tests.
a w/c = 0.5. Specimens with four 3.2-mm (0.125-inch)-diameter holes in
coating.
b w/c = 0.45. ECR systems tested using 3, 6, or 12 specimens with
four 3.2-mm (0.125-inch)-diameter holes in coating and three specimens with 10 3.2-mm
(0.125-inch)-diameter holes in coating.
c w/c = 0.45. ECR systems tested with 16 3.2-mm (0.125-inch)-diameter
holes in coating.
d Six or 12 corrosion initiation specimens also tested.
e Twelve specimens with w/c = 0.45 and three specimens with w/c = 0.35.
f Half without and half with cracks over the test bars.
g Includes three additional specimens with w/c = 0.35 and 10 3.2-mm
(0.125-inch)-diameter holes in coating.
h Three specimens with four 3.2-mm (0.125-inch)-diameter holes in
coating.
As shown in table 8, the rapid macrocell test with mortar-wrapped specimens was used for all multiple protection systems, while the macrocell test with bare bars was not used to evaluate the effects of corrosion inhibitors. The macrocell tests for the bare and mortar-wrapped specimens for the MC system included bars with penetrations through both layers as well as bars in which only the outer layer of epoxy had been penetrated. The coating on each bar in the rapid macrocell tests was penetrated by four 3.2-mm (0.125-inch)-diameter holes, representing damage to 1.0 percent of the bar area exposed to solutions in these tests. Six specimens were used for each system.
Southern exposure, cracked beam, and corrosion initiation tests were used to compare the performance of corrosion-protection systems cast in concrete. A w/c ratio of 0.45 was used in all cases. In addition, concrete with a w/c ratio of 0.35 was used to evaluate the performance of the corrosion inhibitors (as well as the control specimens) because the corrosion protection provided by calcium nitrite relative to that of other corrosion inhibitors has been observed to improve as the w/c ratio and permeability of the concrete decrease.(47) The coatings on bars in the bench-scale tests were penetrated with 4 or 10 holes with a diameters 3.2 mm (0.125 inches), representing damage to 0.21 or 0.52 percent of the bar surface, respectively.
Linear polarization resistance measurements were performed on a single southern exposure and cracked beam specimen for each configuration and corrosion-protection system in the study (see table 8). The results were used in conjunction with the readings obtained from the macrocell, bench-scale, and field tests to characterize the performance of the corrosion-protection systems.
Field test specimens were used to compare conventional reinforcing steel and conventional ECR steel to epoxy-coated bars with increased adhesion, epoxy-coated bars with corrosion inhibitors, and MC bars with both layers penetrated. Four specimens were included for each category, except there were six epoxy-coated bar specimens with calcium nitrite. Half of the specimens had simulated cracks above top reinforcing bars. ECR was cast with 16 3.2-mm (0.125-inch)-diameter holes in the coating, representing 0.24 percent of the bar surface.
Following the rapid macrocell, bench-scale, and field tests, specimens were photographed to record cracking and corrosion products visible on the exterior of concrete or mortar and on reinforcing steel and surrounding cementitious materials after the removal of concrete or mortar. Following the bench-scale and field tests, coatings were evaluated for disbondment, and concrete samples were taken to analyze for chloride contents.