U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| REPORT |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
| Publication Number: FHWA-HRT-14-086 Date: November 2014 |
Publication Number: FHWA-HRT-14-086 Date: November 2014 |
QUANTITATIVE ANALYSIS OF ASPHALT BINDERS FOR PHOSPHORIC ACID
X-ray fluorescence spectroscopy (XRF) is an analytical technique by which all the elements in the periodic table from sodium to uranium can be quantitatively and rapidly detected with minimal sample preparation. Test samples are irradiated with an X-ray beam, and the resulting spectrum can be used to provide quantitative information on each element present.
The use of XRF to quantitatively determine the amount of phosphoric acid contained in asphalt binders was developed by Puzic et al.(3) The method has been refined by Reinke et al.(4)
Samples are placed in cups consisting of two concentric polypropylene rings over which a thin plastic film is stretched like a drum skin. The X rays are able penetrate the film with no attenuation of the beam. Initially, 6-micron Mylar® polyester film was chosen for its strength. It was discovered that it contains the equivalent 0.1-percent phosphoric acid and was discarded in favor of polypropylene, which contains none. Pictures of the inverted cups are shown in figure 1 and figure 2. A drop of water has been placed on the film of the empty cup in figure 1 to make the film visible. Warm asphalt is poured into the empty cup while it is sitting on a ¼-inch thick aluminum plate. The plate acts as a heat sink and prevents the heat of the asphalt from melting the plastic film. The asphalt temperature is not critical; the asphalt just needs to be molten. Typical pouring temperatures are 140 °C.
The plastic cup, filled with asphalt, is then placed inside a stainless steel cup holder (figure 3), which is placed inside the spectrometer (figure 4). Each sample takes 20 to 25 min to run. The program runs automatically, and the spectrometer is capable of analyzing up to 52 samples unattended.

Figure 1. Photo. XRF cup (inverted) with plastic membrane.

Figure 2. Photo. XRF cup (inverted) filled with asphalt.

Figure 3. Photo. Steel XRF cup holder.

Figure 4. Photo. Interior of XRF spectrometer.
All asphalt binders contain a significant amount of sulfur; they do not contain phosphorus. Because sulfur and phosphorus are next to each other in the periodic table they have very similar XRF energies. The major Kα peak energy for phosphorus is 2.013 KeV and for sulfur 2.307 KeV. This proximity causes the peaks in the XRF spectrum from these two elements to overlap. Because the amount of sulfur in asphalt is very much higher than the amounts of phosphoric acid typically used for modification, the sulfur peak is very much larger. It overwhelms the phosphorus peak. This negatively affects the accuracy of the analysis. The XRF spectrometer software "sees" a phosphorus peak when none may be present. This phenomenon is clearly shown in figure 6, the XRF spectrum of asphalt AAB-1. At the energy level of approximately 2.0 KeV on the x-axis, the software has labeled the spectrum P-Ka1 indicating a phosphorus Kα peak when none is present. The software is using the intensity of the first part of the sulfur peak and interprets it incorrectly. Ninety samples of unmodified asphalt binders showed a phosphoric acid level of 0 to 0.2 percent when we know that none was actually present. Compare this with figure 5, the XRF spectrum of the same asphalt, AAB-1, modified with 1 percent of 105-percent phosphoric acid where the phosphorus peak can be clearly seen. There is no fixed detection limit. The results may also depend on the spectrometer used; however, these results suggest that XRF analyses indicating the presence of low levels of approximately 0.2 percent or less might be misleading.
The accuracy of the phosphoric acid analysis was improved markedly by entering into the spectrometer software phosphoric acid calibration standards, the known sulfur content of the binder used. The sulfur levels in the binders were determined using XRF. Accuracy was improved further by using the published sulfur contents of the SHRP reference binders.(5)
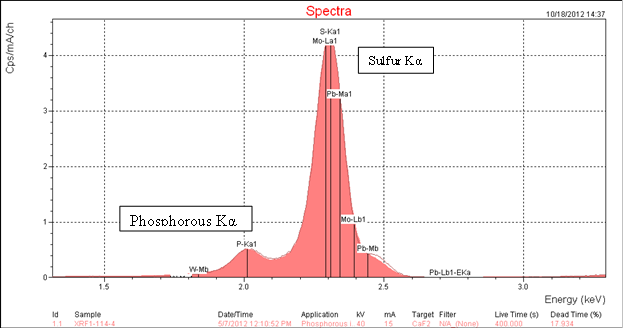
Figure 5. Chart. XRF spectrum of asphalt AAB-1 modified with 1-percent of 105 percent superphosphoric acid.
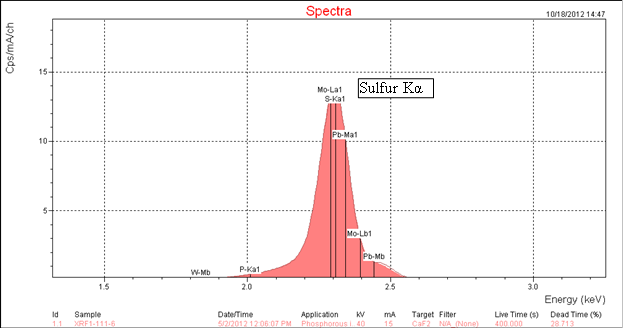
Figure 6. Chart. XRF spectrum of asphalt AAB-1.
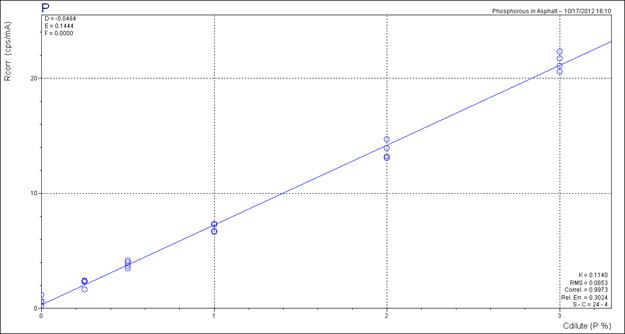
Figure 7. Chart. XRF Calibration chart for phosphoric acid.
Figure 8 shows that the XRF signals of the four SHRP reference asphalts at zero phosphoric acid addition differ slightly.
Figure 9 shows the same data corrected for the difference in zero acid addition.
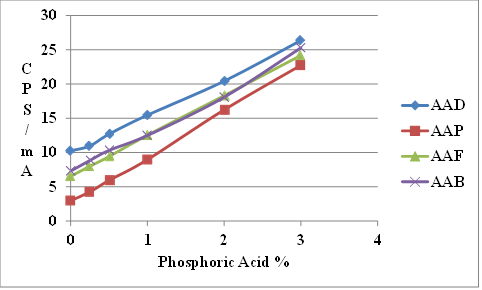
Figure 8. Chart. Plot of XRF signal of SHRP reference asphalts modified with superphosphoric acid content.
The XRF up to 1-percent acid modification is the same for the four asphalts. However, at higher modification levels, the curves diverge, indicating some asphalt dependency. Test results were found to be less accurate at higher modification levels as shown in figure 10.
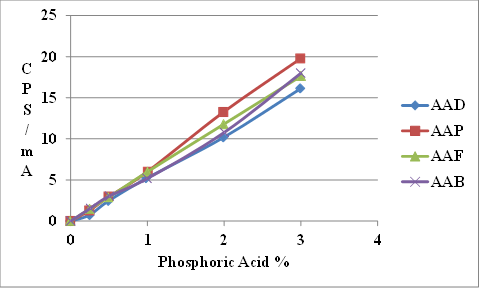
Figure 9. Chart. X-ray fluorescence of SHRP reference asphalts modified with superphosphoric acid corrected for baseline fluorescence.
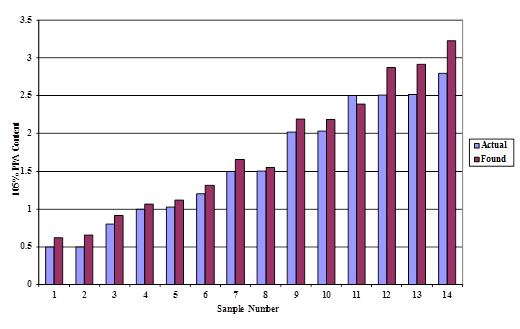
Figure 10. Chart. Accuracy of PPA analysis using XRF.
A SIMPLE QUALITATIVE TEST TO DETECT THE PRESENCE OF PHOSPHORIC ACID IN ASPHALT BINDERS
Because not all State agencies have access to XRF spectrometers, a simple procedure, the "Susan P. Needham Test," was developed. It is a very simple technique; it requires no special equipment-just the use of a few simple chemicals. The test is very sensitive, and care must be taken that the equipment used and chemical used do not contain phosphates. This is particularly true for the use of metal cans that contain a phosphate film on the surface because they will give a positive result. The test has been submitted to AASHTO and is published as provisional test method TP 78-09, "Detecting the Presence of Phosphorus in Asphalt Binder." The following describes the reagents and procedures used in the test method.
Description:
Identification:
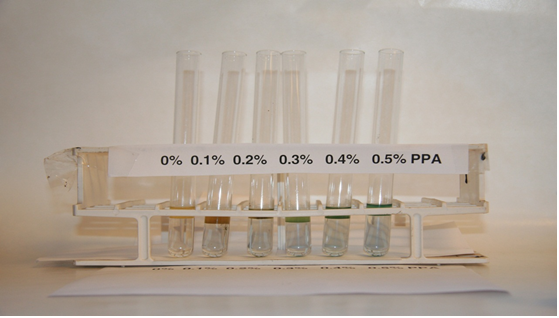
Figure 11. Photo. Phosphoric acid detected by the blue color developed in the Susan P. Needham test.
SATURATE, AROMATIC, RESIN, AND ASPHALTENE ANALYSIS OF ASPHALT BINDERS MODIFIED WITH PHOSPHORIC ACID
Solvent separation of asphalt binders (saturate, aromatic, resin, and asphaltene (SARA) analysis) was accomplished using Chromarods® (thin layer chromatography) run on the Iatroscan® TH-10 hydrocarbon analyzer. This method results in the separation of the four operationally defined fractions inherently present in all petroleum-derived asphalt and asphaltic residuals, namely are asphaltenes, resins, aromatics, and saturates.
Asphaltenes are the viscosity builders in asphalt. They are black amorphous solids that contain the bulk of the heteroatoms (nitrogen, sulfur, and oxygen) found in asphalt. Trace elements, such as nickel and vanadium, are also present. Asphaltenes are highly polar aromatic materials of aggregated molecular weights of 750 (number average), and constitute approximately 5 to 25 percent of the weight of asphalt.
Resins (polar aromatics) are dark-colored, solid or semi-solid, very adhesive fractions of relatively high molecular weight present in the maltenes. They are the dispersing agents or peptizers for the asphaltenes, and the ratio of resins to asphaltenes governs, to a degree, the sol- or gel-type character of asphalts. Resins separated from bitumen are found to have molecular weights of 800 to 2,000 (number average) but there is a wide molecular distribution. This component constitutes 15 to 25 percent of the weight of asphalts.
Cyclics (naphthenic aromatics) comprise the compounds of lowest molecular weight in bitumen and represent the major portion of the dispersion medium for the peptized asphaltenes. They constitute 45 to 60 percent by weight of the total asphalt and are dark viscous liquids. They are compounds with aromatic and naphthenic aromatic nuclei with side chain constituents and have molecular weights of 500 to 900 (number average).
Saturates comprise predominately the straight-and-branched-chain aliphatic hydrocarbons present in bitumen, together with alkyl naphthenes and some alkyl aromatics. The average molecular weight range is approximately similar to that of the cyclics, and the components include the waxy and non-waxy saturates. This fraction forms 5 to 20 percent of the weight of asphalts.
The test method used was kindly provided by Dr. Gaylon Baumgardner of Ergon® Inc. A copy of the method is provided in the appendix.
The four test asphalts, AAD-1, AAK-1, AAM-1, and ABM-1 were dosed with the equivalent of 1-percent acid. For phosphorus pentoxide, the stoichiometry calculates to 0.75 percent. The samples were conditioned overnight at 165 °C. Separation was carried out according the aforementioned procedure. The results are shown in figure 12 to figure 15 .
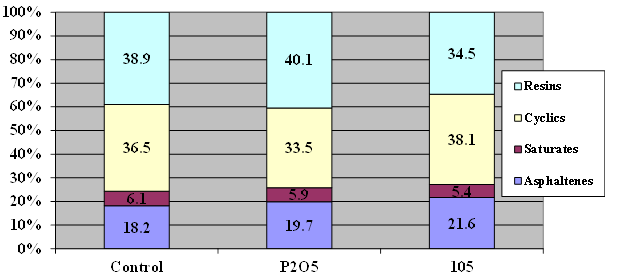
Figure 12. Chart. Results of SARA fractionation of AAD-1 modified with 1 percent of 105-percent superphosphoric acid or 0.75-percent phosphorus pentoxide.
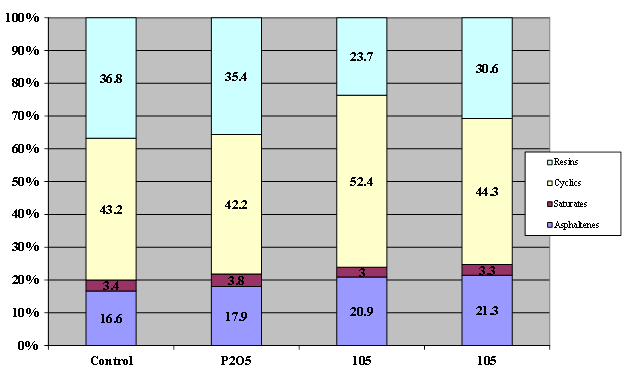
Figure 13. Chart. Results of SARA fractionation of asphalt AAK-1 modified with 1 percent of 105-percent superphosphoric acid or 0.75-percent phosphorus pentoxide.
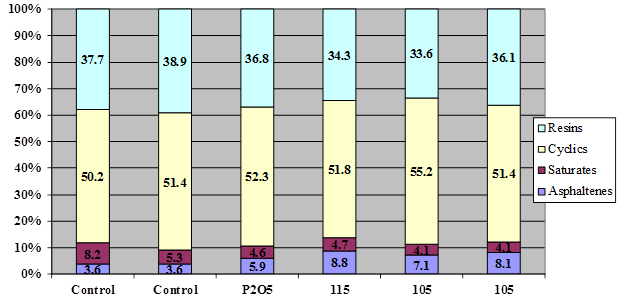
Figure 14. Chart. Results of SARA fractionation of asphalt AAM-1 modified with 1 percent of 115-percent PPA, 1 percent of 105-percent superphosphoric acid, or 0.75-percent phosphorus pentoxide.
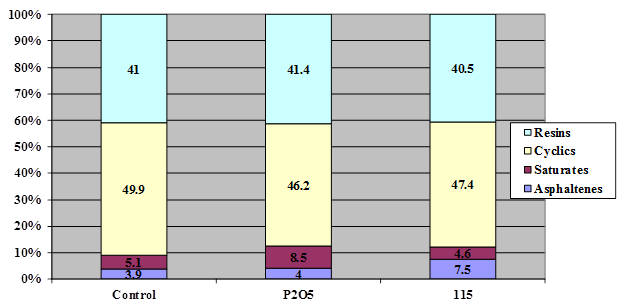
Figure 15. Chart. Results of SARA fractionation of asphalt ABM-1 modified with 1 percent of 115-percent PPA or 0.75-percent phosphorus pentoxide.
The Iatroscan® technique is very sensitive to temperature, humidity, and time. Test results in the Turner Fairbank Highway Research Center (TFHRC) chemistry laboratory showed a high degree of variability because, at the time of this research, the building temperature and humidity were not well controlled. This variability precluded the detection of any trend at low levels of acid modification in the components separated by the technique. The tests were repeated, with higher levels of acid than would be expected to be used in practice, to see whether a definite trend could be determined.
Samples of the four SHRP test asphalts were dosed with 115-percent phosphoric acid at acid levels from 1 to 4 percent (based on 100-percent acid) and the samples aged overnight at 165 °C. The variability of the technique is evident from the shapes of the curves shown in the following four charts (figure 16 through figure 20). The data do, however, illustrate trends.
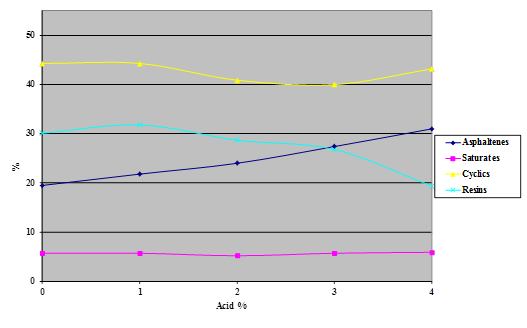
Figure 16. Chart. Results of SARA separation of asphalt AAD-1 modified with 115-percent phosphoric acid.
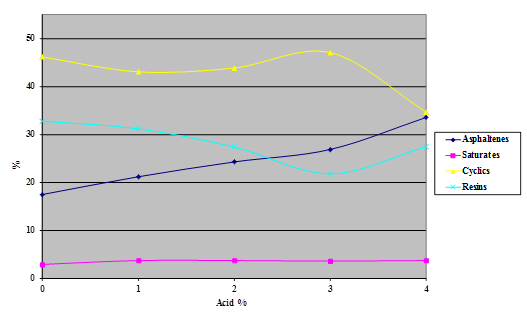
Figure 17. Chart. Results of SARA separation of asphalt AAK-1 modified with 115-percent phosphoric acid.
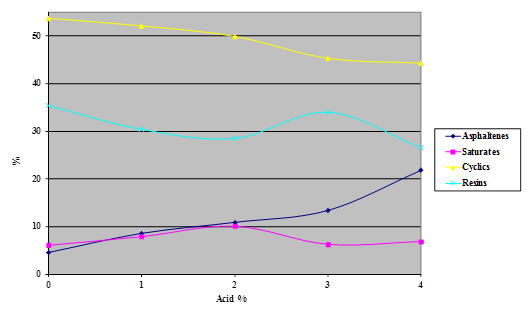
Figure 18. Chart. Results SARA separation of asphalt AAM-1 modified with 115-percent phosphoric acid.
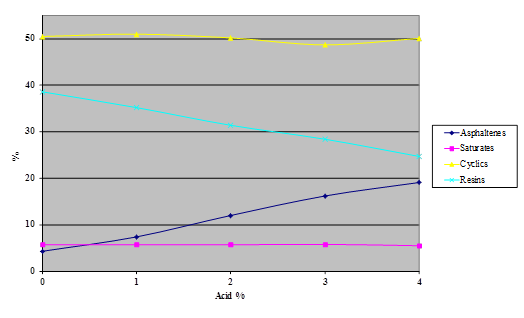
Figure 19. Chart. Results of SARA separation of asphalt ABM-1 modified with 115-percent phosphoric acid.
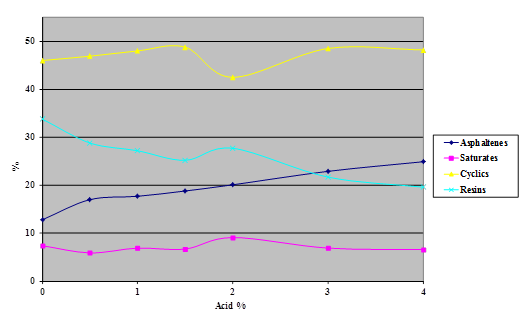
Figure 20. Chart. Results of SARA separation of B6317 Venezuelan asphalt modified with 115-percent phosphoric acid.
The following findings resulted from the SARA analysis:
HOW DOES THE PHOSPHORIC ACID REACT WITH THE BINDER?
A small amount of the asphaltenes and heptane-insoluble fractions from the initial solvent separations was analyzed using the energy dispersive spectrometry attachment to the Amray scanning electron microscope. This is purely a qualitative test. It showed that the heptane-insoluble fraction contained phosphorus while the heptane-soluble maltene fraction contained none.
This was confirmed by Liquid State 31P nuclear magnetic resonance (NMR) spectra. The spectrum for the heptane-insoluble fraction (asphaltenes) shown in figure 21 clearly shows the peak due to the presence of phosphorus. In figure 22, the NMR spectrum for the heptane-soluble fraction (maltenes) has no phosphorus peak.
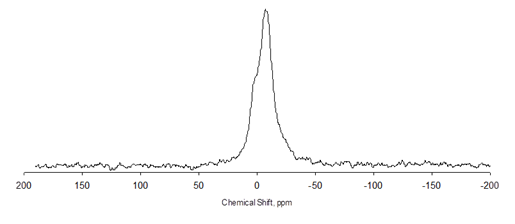
Figure 21. Chart. NMR spectrum of heptane-insoluble fraction of phosphoric acid-modified asphalt.
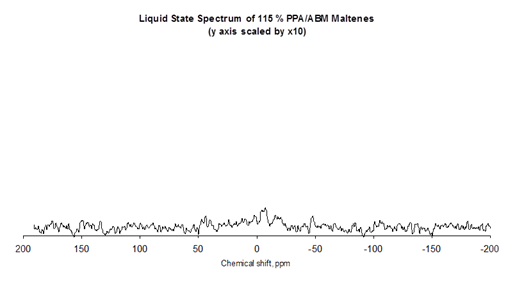
Figure 22. Chart. NMR spectrum of heptane soluble fraction of phosphoric acid-modified asphalt.
MAJOR CONCLUSIONS FROM CHAPTER 2, ANALYTICAL METHODS