U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| REPORT |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
| Publication Number: FHWA-HRT-14-086 Date: November 2014 |
Publication Number: FHWA-HRT-14-086 Date: November 2014 |
EFFECT OF WATER ON ASPHALT MASTICS WITH AND WITHOUT PHOSPHORIC ACID
Phosphoric acid is a very hydrophilic material. Some concerns were expressed that its use may negatively affect the moisture resistance of asphalt mixes. Tests were performed that involved immersing samples of mastics and binders in water for extended periods of time. This condition is rarely found in practice, and therefore represents an extreme case and should not be interpreted as an indication of what would happen on a real highway.
To measure the moisture resistance, mastics containing 50-percent aggregate fines (sand, diabase, gravel, and montmorillonite) were cast into silicone rubber molds in the shape of either direct tension dog bones or Bending Beam Rhometer (BBR) samples. (These were the only silicon rubber molds available.) Montmorillonite was used because it is water-sensitive expansive clay. The presence of such materials can have a deleterious effect on asphalt pavements. The asphalt binder used (B6317) was supplied by Citgo® and was a blend of 60-percent Bachaquero and 40-percent Menemota 21.
The mastic samples were weighed and then immersed in a water bath at 7 °C. At intervals over the next 105 days, the dog bones were dried with a paper towel, weighed, and the amount of water absorbed calculated. The results are shown in the figure 42 through figure 45. The effect on the stiffness of soaked specimens of Citgo® asphalt are shown in figure 46.
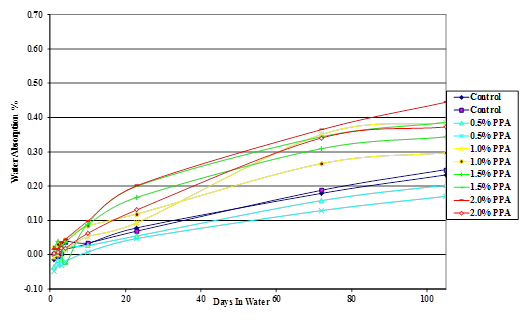
Figure 42. Chart. Plot of moisture absorption of Citgo® 50-percent asphalt/sand mastic modified with 115-percent phosphoric acid.
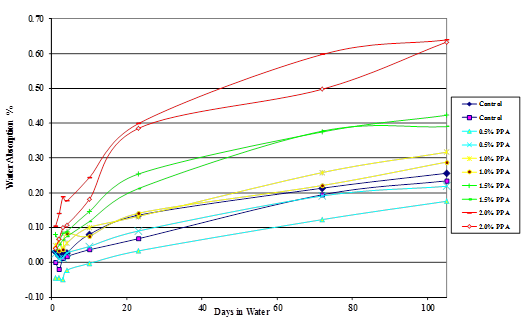
Figure 43. Chart. Plot of moisture absorption of Citgo® 50-percent asphalt/diabase mastic modified with 115-percent phosphoric acid.
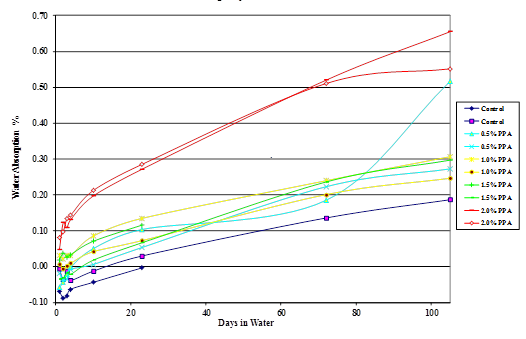
Figure 44. Chart. Moisture absorption of Citgo®50-percent asphalt/gravel mastic modified with 115-percent phosphoric acid.
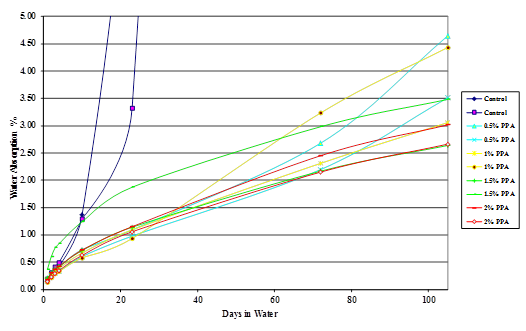
Figure 45. Chart. Moisture absorption of Citgo® 50-percent asphalt/montmorillonite mastic modified with 115-percent phosphoric acid.
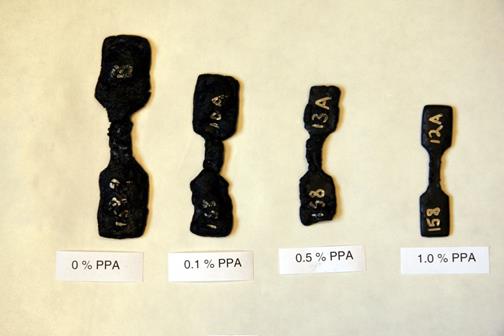
Figure 46. Chart. Fifty-percent montmorillonite asphalt binder mastic after water immersion for 105 days.
EFFECT OF WATER ON NEAT ASPHALT BINDERS
Similar tests to those described above were carried out using BBR beams of neat Venezuelan asphalt (B6317) modified with 115-percent phosphoric acid at levels up to 4 percent. The results are shown in figure 47 indicate the following:
Most samples, including the unmodified control, lost 0.1- to 0.25-percent weight almost immediately. These samples subsequently gained weight and ultimately had an overall weight gains. This suggests the possibility that water-soluble materials are initially being extracted from the asphalt itself; however, no attempt was made to identify the nature of this material.
The control, 0.5- and 1-percent modified samples all showed approximately the same level of increase in weight after 126 days of immersion in water. This weight gain was between 0 and 0.1 percent.
Long immersions and increasing phosphoric acid levels resulted in higher levels of water absorption.
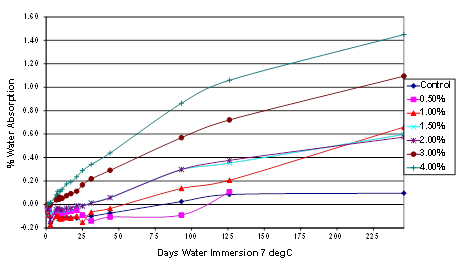
Figure 47. Chart. Plot of water absorption of Citgo®
The effect of this water absorption on the stiffness of the Citgo® asphalt was examined; the results are shown in figure 48.
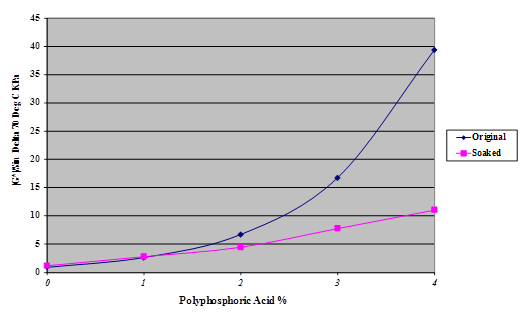
Figure 48. Chart. Plot of stiffness of phosphoric acid modified Citgo® asphalt after 245 days of water immersion.
As mentioned earlier, phosphoric acid is a very hydrophilic material; it is very water soluble. To determine whether there is a risk of rain leaching phosphoric acid from a pavement, a number of soaking tests were devised. Because there is no standard test to determine leachates from HMA pavments, a test protocol was established for comparison purposes. The amount of phosphoric acid extracted from a pavement on a daily basis is likely to be below the limits of detection. To determine whether this could become an issue over a period of time, the following procedure was used to test the likelihood of this event.
The test consisted of immersing gyratory specimens in distilled water contained in clean high density polyethylene (HDPE) buckets. Periodically, the phosphate content of the water over 245 days was measured. This is an extreme case-the specimens were completely immersed in water whereas pavements rarely are.
Gyratory specimens 6 by 4.5 inches were made using the standard TFHRC Accelerated Loading Facility dense coarse graded 12.5-mm nominal maximum aggregate size Superpave mix with a 5-percent binder content. The aggregate was a diabase. Each core contained approximately 4,885 g of aggregate and 258 g of binder (Citgo® binder referenced earlier). The binder was modified with levels of PPA from 0 to 4 percent. The cores were placed in new clean HDPE buckets to which 2.5 L of distilled water were added; this amount was sufficient to cover the specimens. The phosphoric acid content of the water was measured over a period of 245 days using ion exchange chromatography (Dionex™ ICS-2000). The calculations were made assuming the phosphate was present in the water as orthophosphoric acid.
To determine whether there was any asphalt or aggregate dependency, a second suite of samples was tested. In this case, two asphalt binders were used with two different aggregates, namely a Lion Oil (B6367) and BP Whiting (B6364) binder and diabase and Georgia granite aggregates. To gain some insight on the effect of air void content, the samples were tested as both gyratory specimens and uncompacted loose mix. Six levels of PPA modification were chosen; 0, 0.5, 0.75, 1.0, 1.5, and 3 percent.
The results are presented graphically in figure 49 through figure 52, and the percent of the added phosphoric acid extracted is given in table 10, which shows that only a very small percent of the phosphoric acid is extracted after 245 days in water.
The amount of phosphate leached from the gyratory specimens made with diabase or granite aggregates was the same for both the Lion Oil and BP Whiting binders although extraction levels for the granite specimens was significantly higher, some being leached even at a phosphoric acid modification level as low as 0.5 percent. As would be expected, more phosphate was leached from the loose mixes compared with the compacted gyratory specimens. Slightly more phosphate was leached from the loose mix made with the Lion Oil binder. The results for the loose mixes are presented in figure 53 and figure 54.
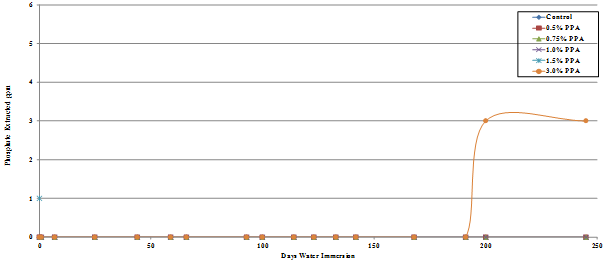
Figure 49. Chart. Plot of phosphate extracted from Lion Oil binder diabase aggregate gyratory specimen.
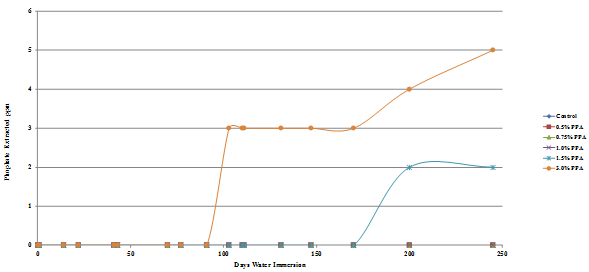
Figure 50. Chart. Plot of phosphate extracted from BP Whiting binder diabase aggregate gyratory specimen.
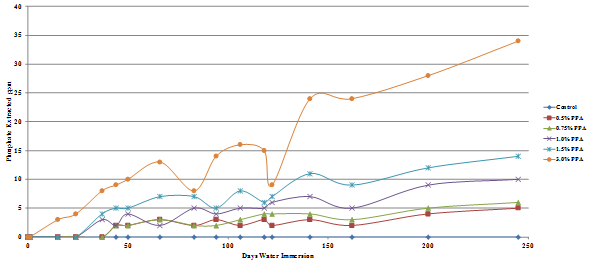
Figure 51. Chart. Plot of phosphate extracted from Lion Oil binder granite aggregate gyratory specimen.
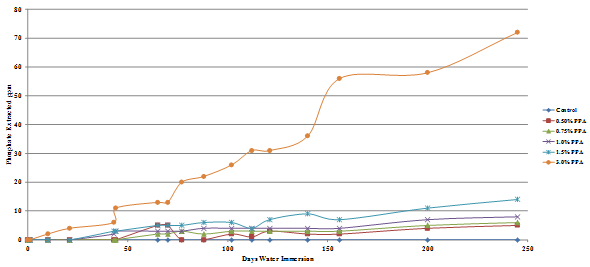
Figure 52. Chart. Plot of phosphate extracted from BP Whiting binder granite aggregate gyratory specimen.
Table 10 . Percentage of the added phosphoric acid extracted after 245 days of immersion in water.
PPA Percent |
B6317/ Diabase |
Lion Oil/ Diabase |
BP/ |
Lion Oil/ Granite |
BP Granite |
Lion Oil/ Diabase |
BP/ Diabase |
|---|---|---|---|---|---|---|---|
Gyratory |
Gyratory |
Gyratory |
Gyratory |
Gyratory |
Loose Mix |
Loose Mix |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0.5 |
0 |
0 |
0 |
0.39 |
0.39 |
0.85 |
0 |
0.75 |
0 |
0 |
0 |
0.31 |
0.31 |
- |
0 |
1.0 |
0.14 |
0 |
0 |
0.39 |
0.31 |
0.66 |
0 |
1.5 |
0.14 |
0 |
0.05 |
0.36 |
0.36 |
0.72 |
0.18 |
2.0 |
0.28 |
0 |
- |
- |
- |
- |
- |
3.0 |
0.78 |
0.04 |
0.06 |
0.44 |
0.93 |
0.54 |
0.39 |
4.0 |
1.63 |
- |
- |
- |
- |
- |
- |
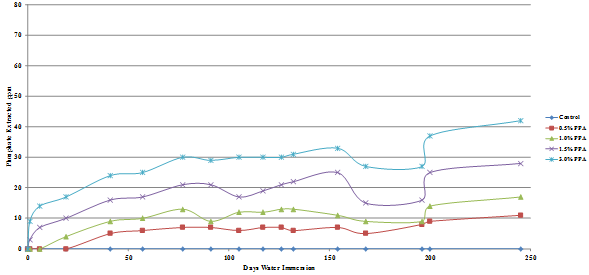
Figure 53. Chart. Plot of phosphate extracted from Lion Oil binder diabase aggregate loose hot mix.
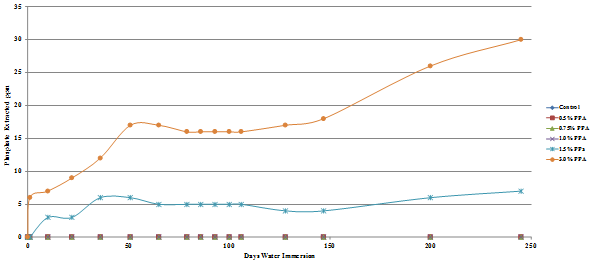
Figure 54. Chart. Plot of phosphate extracted from BP Whiting binder diabase aggregate loose hot mix.
EFFECT OF PHOSPHORIC ACID MODIFICATION IN THE USE OF ANTISTRIP ADDITIVES
One of the preconceived notions on the use of phosphoric acid as an asphalt binder was that it could not be used with liquid amine antistrip additives because it is an acid and would react with the basic components in the additives. It was also proposed that nonamine liquid antistrip additives, for example 2-ethylhexyl phosphate, could be used because they would not react chemically with the phosphoric acid.
To confirm that phosphoric acid reacts chemically with liquid amine antistrip additives, samples were dissolved in ethanol (they are not soluble in water) and titrated with phosphoric acid using a standard acid/base indicator. One g of AD-HERE® LOF 65-00 was found to be equivalent to 0.49 g PPA and AD-HERE® LA-2 to 0.57 g PPA. If the binder contains 0.5 percent of antistrip additive and 1 percent of PPA, then the PPA is in excess, with about 25 percent being neutralized by the amine.
In this study, a number of commercially available liquid amine antistrip additives were evaluated with several different aggregates and binders to test the validity of these concerns and the effect that phosphoric acid modification would have on moisture resistance of HMA pavements.
The antistrip additives were the following:
The aggregates used were sandstone (Keystone Aggregates, MD), limestone (H.B. Mellot, MD), and granite from Georgia (unknown origin). The binder was supplied by Citgo®.
The stripping tests were carried out using the Hamburg wheel tracker. The water temperature was 50 °C and the pass/fail criterion 20,000 cycles with a maximum rut depth of 12.5 mm. Results are shown in figure 55 through figure 69.
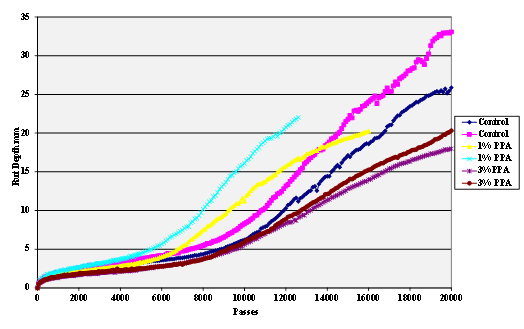
Figure 55. Chart. Hamburg rut test of Citgo® asphalt and sandstone aggregate.
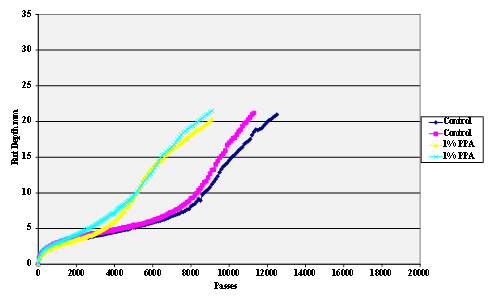
Figure 56. Chart. Hamburg rut test of Citgo® asphalt and limestone aggregate.
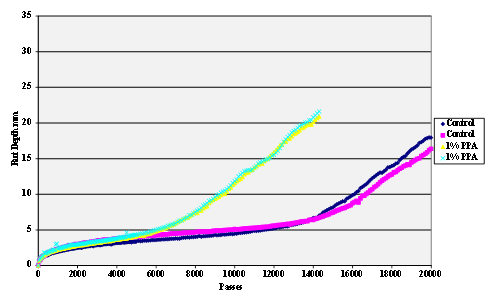
Figure 57. Chart. Hamburg rut test of Citgo® asphalt and granite aggregate.
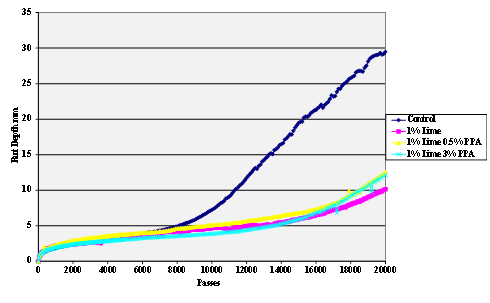
Figure 58. Chart. Hamburg rut test of Citgo® asphalt and lime-treated sandstone aggregate.
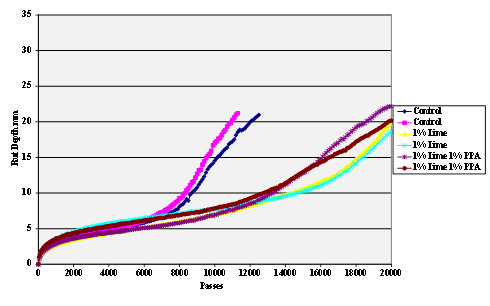
Figure 59. Chart. Hamburg rut test of with Citgo® asphalt and lime-treated limestone aggregate.
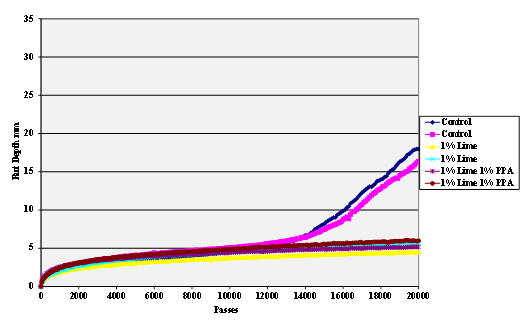
Figure 60. Chart. Hamburg rut test of Citgo® asphalt and lime-treated granite aggregate.
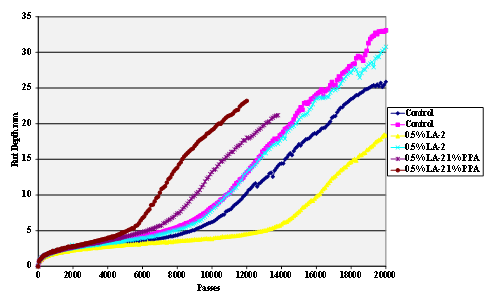
Figure 61. Chart. Hamburg rut test of Citgo® asphalt AD-HERE® LA-2 antistrip-treated sandstone aggregate.
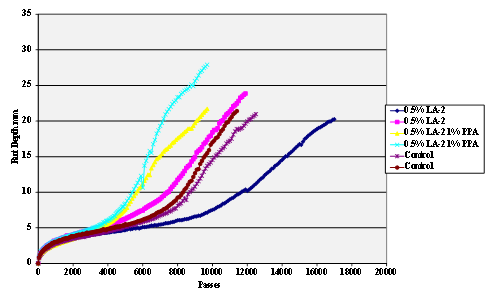
Figure 62. Chart. Hamburg rut test of Citgo® asphalt AD-HERE® LA-2 antistrip-treated limestone aggregate.
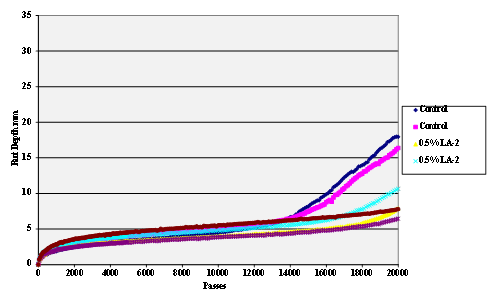
Figure 63. Chart. Hamburg rut test of Citgo® asphalt AD-HERE® LA-2 antistrip-treated granite aggregate.
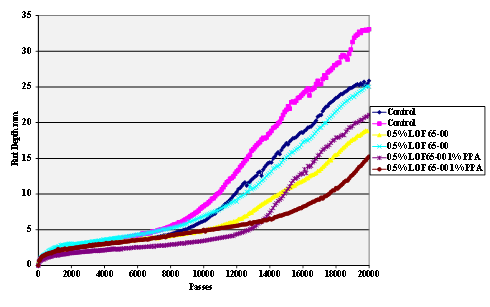
Figure 64. Chart. Hamburg rut test of Citgo® asphalt AD-HERE® LOF 65-00 antistrip-treated sandstone aggregate.
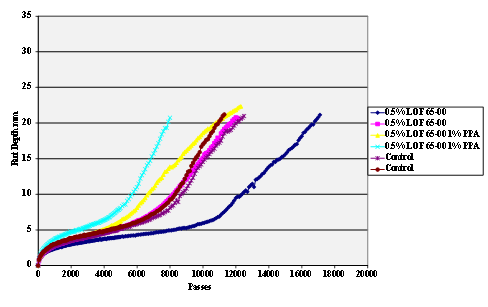
Figure 65. Chart. Hamburg rut test of Citgo® asphalt AD-HERE®
LOF 65-00 antistrip-treated limestone aggregate.
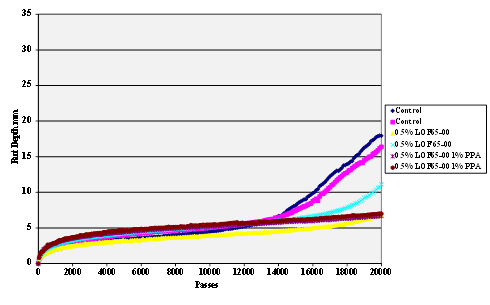
Figure 66. Chart. Hamburg rut test of Citgo® asphalt AD-HERE® LOF 65-00 antistrip-treated granite aggregate.
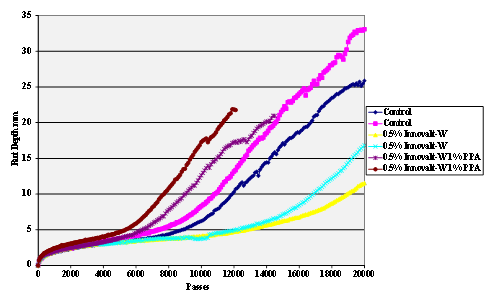
Figure 67. Chart. Hamburg rut test of Citgo® asphalt Innovalt®
-W antistrip-treated sandstone aggregate.
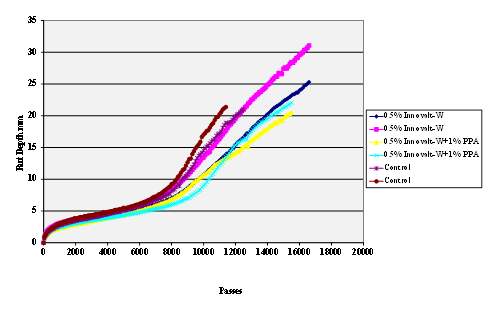
Figure 68. Chart. Hamburg rut test of Citgo® asphalt Innovalt®
-W antistrip-treated limestone aggregate.
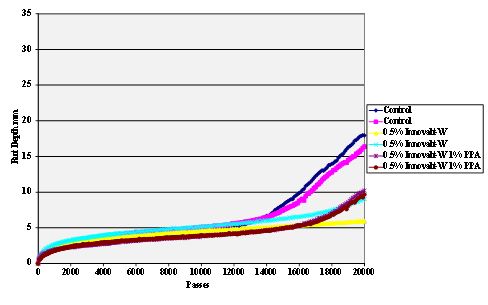
Figure 69. Chart. Hamburg rut test of Citgo® asphalt Innovalt®
-W antistrip-treated granite aggregate.
The data are summarized table 11 . The results are compared with the control for each aggregate. The results are the average of the duplicate specimens that were tested. Bold indicates the samples performed better than the control; those in italic were worse than the control.
Table 11 . Summary of Hamburg rut testing results with antistrip additives.
Aggregate |
Additive |
Cycles to 12.5 mm |
Failure Cycles |
Depth at 20,000 Cycles mm |
|---|---|---|---|---|
Sandstone |
Control |
12,500 |
- |
29 |
1 Percent Phosphoric Acid |
9,500 |
14,300 |
- |
|
3 Percent Phosphoric Acid |
14,550 |
- |
19 |
|
AD-HERE® LA-2 |
14,350 |
- |
24 |
|
AD-HERE® LA-2 + 1 Percent Phosphoric Acid |
8,700 |
13,000 |
- |
|
AD-HERE® LOF 65-00 |
15,150 |
- |
22 |
|
AD-HERE® LOF 65-00 + 1 Percent Phosphoric Acid |
17,300 |
- |
18 |
|
1 Percent Lime |
- |
- |
10 |
|
1Percent Lime+0.5 percent Phosphoric Acid |
- |
- |
12 |
|
1 percent Lime+3 percent Phosphoric Acid |
- |
- |
12 |
|
Innovalt®-W |
18,200 |
- |
14 |
|
Innovalt®-W+1 Percent Phosphoric Acid |
9,300 |
13,200 |
- |
|
Limestone |
Control |
9,200 |
11,800 |
- |
1 Percent PPA |
5,800 |
9,050 |
- |
|
AD-HERE® LA-2 |
10,600 |
14,400 |
- |
|
AD-HERE® LA-2+1% Phosphoric Acid |
6,100 |
9,700 |
- |
|
AD-HERE® LOF 65-00 |
11,250 |
14,650 |
- |
|
AD-HERE® LOF 65-00 + 1 percent Phosphoric Acid |
6,900 |
10,100 |
- |
|
1 percent Lime |
17,000 |
- |
19 |
|
1 percent Lime+0.5 percent Phosphoric Acid |
14,700 |
- |
21 |
|
Innovalt®-W |
10,350 |
16,550 |
- |
|
Innovalt®-W+1% Phosphoric Acid |
11,150 |
15,450 |
- |
|
Granite |
Control |
17,450 |
- |
17 |
1 Percent Phosphoric Acid |
10,250 |
14,250 |
- |
|
AD-HERE® LA-2 |
- |
- |
9 |
|
AD-HERE® LA-2 + 1Percent Phosphoric Acid |
- |
- |
7 |
|
AD-HERE® LOF 65-00 |
- |
- |
9 |
|
AD-HERE® LOF 65-00 + 1 Percent Phosphoric Acid |
- |
- |
7 |
|
1 Percent Lime |
- |
- |
5 |
|
1 Percent Lime + 0.5 Percent Phosphoric Acid |
- |
- |
6 |
|
Innovalt®-W |
- |
- |
8 |
|
Innovalt®-W+1 Percent Phosphoric Acid |
- |
- |
10 |
Bold indicates sample performed better than the control sample
Italic indicates sample performed worse than the control sample
- Indicates not applicable