U.S. Department of Transportation
Federal Highway Administration
1200 New Jersey Avenue, SE
Washington, DC 20590
202-366-4000
Federal Highway Administration Research and Technology
Coordinating, Developing, and Delivering Highway Transportation Innovations
| REPORT |
| This report is an archived publication and may contain dated technical, contact, and link information |
|
| Publication Number: FHWA-HRT-13-085 Date: October 2013 |
Publication Number: FHWA-HRT-13-085 Date: October 2013 |
In addition to expansion measurements, a limited petrographic analysis is performed on companion CPT samples as a complementary assessment of the progression of damage. Companion samples for petrography were cast from each of the mixes tested by NIRAS and CPT. The petrographic examination relies on the use of a fluorescent stain that can be used to quickly identify the presence of ASR gel. The uranyl acetate staining technique was introduced by Natesaiyer and Hover, and it has also been appended to ASTM C856 Standard Practice for the Petrographic Examination of Hardened Concrete.(20,21) From previous studies, it has been determined that silica gel possesses the capability of adsorption of ions as well as ion exchange. When the ASR gel is formed in concrete, the cations present may include calcium, sodium, and potassium. Through ion exchange, the uranyl ion, in uranyl acetate stain solution, can replace the cations present in the gel. Because the uranyl ion fluoresces green when excited by ultraviolet radiation at 0.00001 inches (254 nm) (UV-C light), the silica gel in concrete can be easily identified with a UV-C light source after staining. However, it has been found that siliceous, not necessarily reactive, aggregates also fluoresce because the silica surface always contains free OH- groups with adsorbed cations, which can be replaced by the uranyl ion.(21) This can cause complications with the analysis of the images because the fluorescence of the aggregate can make it difficult to distinguish between the aggregate and reaction rims. Despite this limitation, the technique is still useful for tagging possibly relevant features in the microstructure, which simplifies the petrography.
Whenever petrography is performed, a 0.5-inch-thick rectangular sample is cut, using a table saw, from the concrete prism. The sample is rinsed briefly with de-ionized water and placed in a fume hood, as a safety precaution to prevent inhalation. The 0.11 N uranyl acetate solution is applied to the freshly cut surface using a pipette and allowed to rest for 1 minute. Next, the surface is thoroughly rinsed with de-ionized water, and the sample is placed under a microscope. A heavy tarp is placed over the microscope instead of using a dark room. A UV lamp is used to illuminate the surface of the sample and a built-in camera (SPOT™ Insight color camera) is used to capture the image from the microscope (Leica® MZ6 stereomicroscope). The initial petrography was conducted using a handheld UV lamp (UVP Model UVSL-14P) and, in an effort to improve image quality, a higher-intensity pen-ray lamp (UVP Model 11SC-1), with short wavelength filter, has been used in the later stages of petrographic examination.
Note that the results presented in this report were obtained on unpolished sections. The loss of the gel was a concern at the start of the petrographic examination, and it was decided to forego polishing to limit this loss. However, in an effort to improve image quality, polishing was tried on an ASR damaged Las Placitas sample. Observation of fluorescing ASR gel suggests that the sample preparation methods used are appropriate for these types of samples. Figure 61 shows a representative unpolished stained section for a concrete prism with a reactive aggregate, and figure 62 shows a polished section for the same concrete prism (different section). The concrete prism is a recast version of Mix 2. Additional specimens for Mix 2 were cast to allow imaging during the initial stages of the reaction. When comparing the images in figure 61 and figure 62, there does not appear to be any evidence of loss of ASR gel. In fact, figure 62 shows a relatively large crack that is still stained after polishing. When comparing the quality of the images, the unpolished section does not appear as clear as the polished section. For the unpolished section, it is difficult to achieve good focus, especially at higher magnifications, resulting in diminished image quality. For the polished section, the image is not only in focus but the stained features are more distinct. In the polished section, it is possible to see microcracks at the edge of the macrocrack. Based on these results, for future petrographic examination, the samples will be polished before staining.
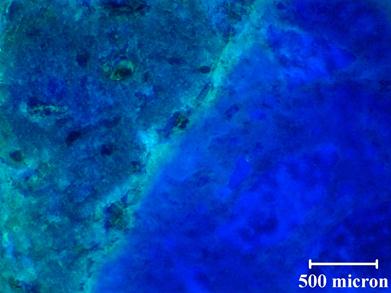
500 microns = 0.19685 inches
Figure 61. Photo. Unpolished stained section
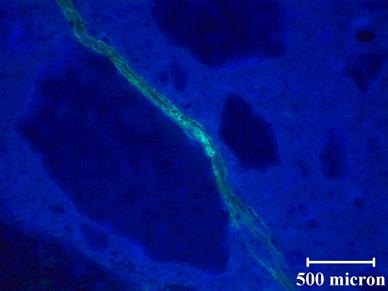
500 microns = 0.19685 inches
Figure 62. Photo. Polished stained section